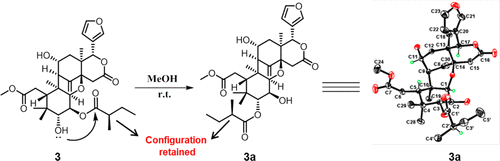
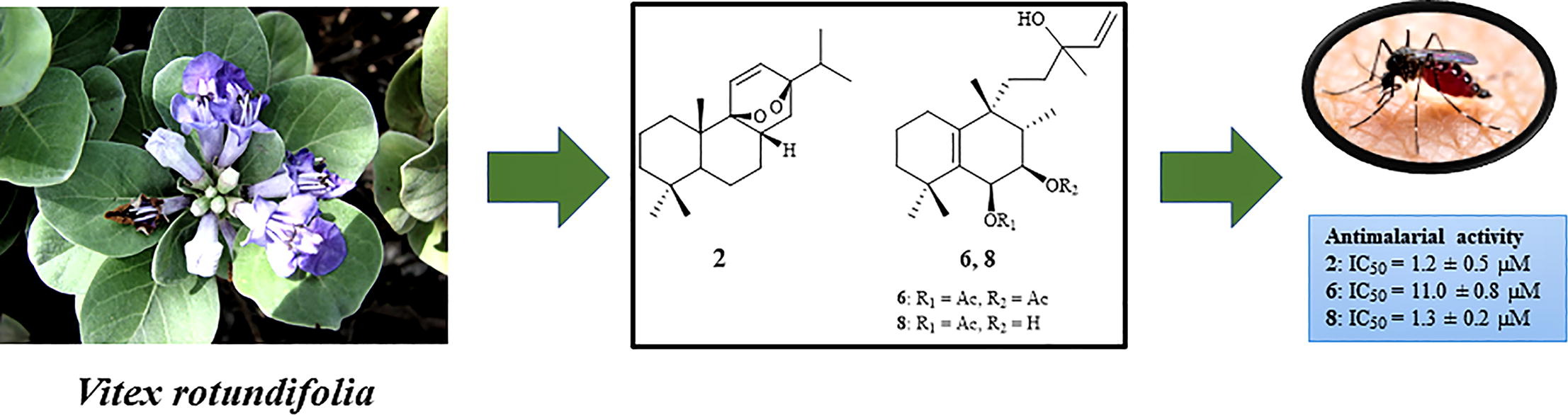
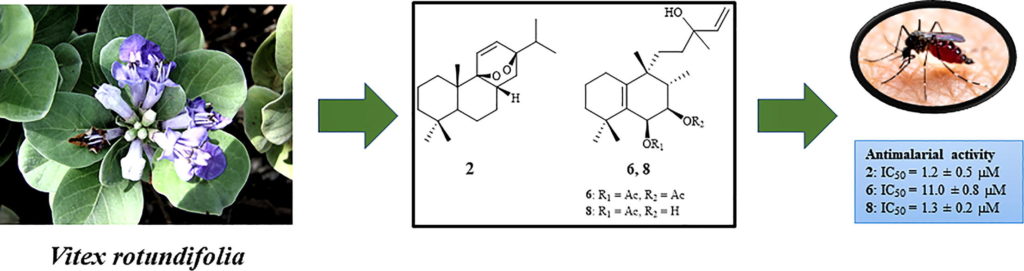
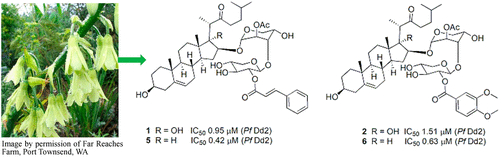
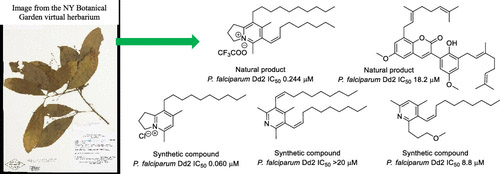
Resistance to some, but not other dimeric lindenane sesquiterpenoid esters is mediated by mutations in a Plasmodium falciparum esterase
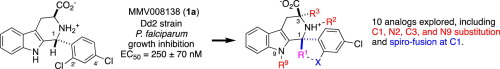
Unique lindenane sesquiterpenoid dimers from Chloranthecae spp. were recently identified with promising in vitro antiplasmodial activity and potentially novel mechanisms of action. To gain mechanistic insights to this new class of natural products, in vitro selection of Plasmodium falciparum resistance to the most active antiplasmodial compound, chlorajaponilide C, was explored. In all selected resistant clones, the half-maximal effective concentration (EC50) of chlorajaponilide C increased >250-fold, and whole genome sequencing revealed mutations in the recently discovered P. falciparum prodrug activation and resistance esterase (PfPARE). Chlorajaponilide C was highly potent (mean EC50 = 1.6 nM, n=34) against fresh Ugandan P. falciparum isolates. Analysis of the structure-resistance relationships revealed that in vitro potency of a subset of lindenane sesquiterpenoid dimers was not mediated by PfPARE mutations. Thus, chlorajaponilide C, but not some related compounds, required parasite esterase activity for in vitro potency, and those compounds serve as the foundation for development of potent and selective antimalarials.
Joshua H Butler, Rodrigo P Baptista, Ana Lisa Valenciano, Bin Zhou, Jessica C Kissinger, Patrick K Tumwebaze, Philip J Rosenthal, Roland Cooper, Jian-Min Yue, Maria Belen Cassera. ACS Infect Dis. 2020 Sep 24. doi: 10.1021/acsinfecdis.0c00487