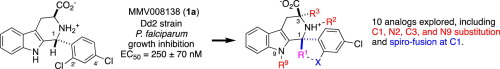
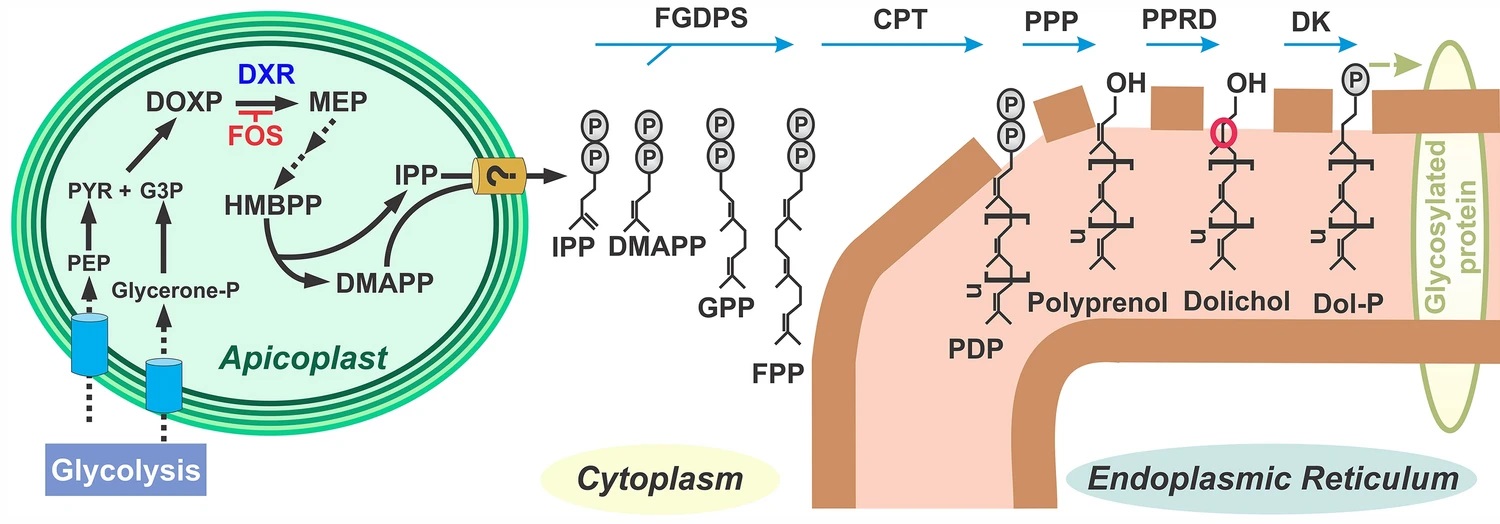
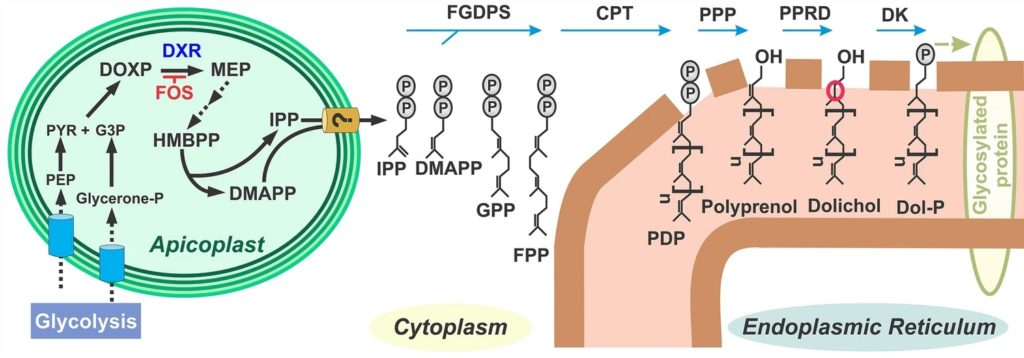
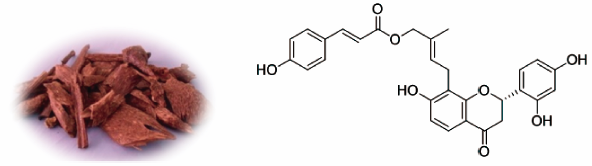
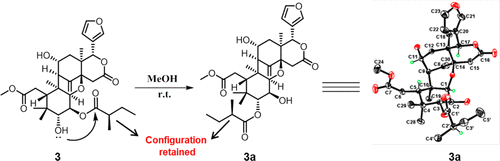
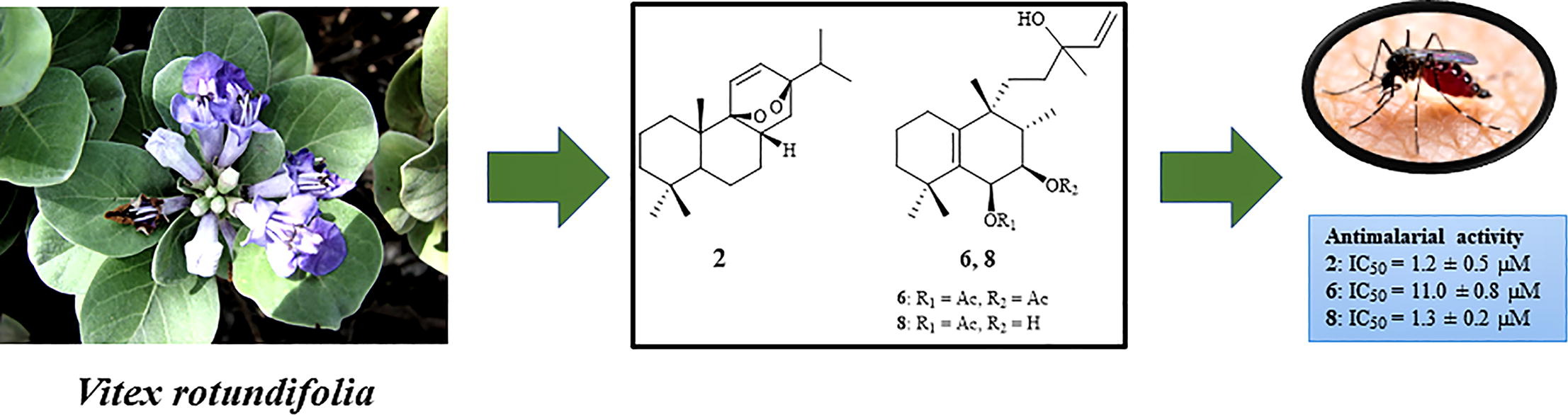
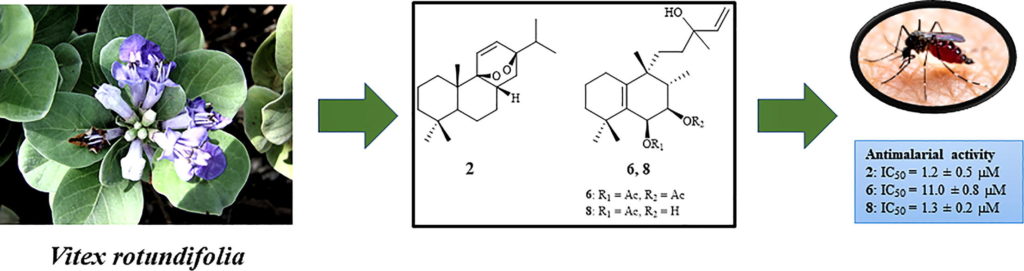
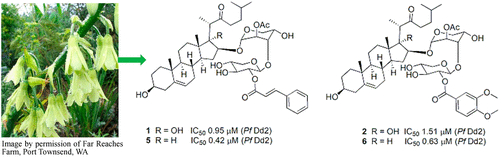
Belen Cassera named parasitology section editor for Current Clinical Microbiology Reports
Last summer, biochemistry associate professor and Center for Tropical and Emerging Global Diseases member Belen Cassera was named the parasitology section editor for Current Clinical Microbiology Reports and has produced her first issue of reviews.
Before former Editor-in-Chief Alan Hudson from Wayne State University School of Medicine stepped down, he recruited Cassera based on a recommendation from Robert Cramer at the Geisel School of Medicine. She met Cramer during an NIH study section.
“Networking is really important if you want to get involved in the editorial side of academic journals,” said Cassera.
In addition to participating in study sections and attending conferences, participating in the peer-review process at journals can also help get your name out there, particularly for senior trainees. She believes that having senior trainees review scientific articles is an important teaching tool.
“Reviewing papers can enhance your own critical thinking,” said Cassera. “Can you ask questions to improve the study or see something missing from the research – reviewing other’s reports helps you to think out the box.”
As section editor, Cassera is responsible for determining the content for the yearly parasitology issue. And she has lofty goals for the parasitology section. She is particularly interested in topics other journals are not covering and that expand the reader’s thoughts on the subject.
“I want readers to have more questions than answers after reading the review,” said Cassera. “I want the articles to spark new ideas.”
For her first issue, she came up with a list of topics and approached researchers that could write on the subjects. However, she is open to unsolicited submissions.
“I would like to include papers for different parasites, but what I’m really looking for are papers that bring new questions to the topic,” said Cassera. “If the scientific community would benefit – if it will lead to new questions or shift our thinking, then I’m interested in it.”
If you have an idea for a possible white paper or review that you think would be a good fit for the parasitology section of Current Clinical Microbiology Reports, then contact Cassera.
Also, be sure to check out the latest reviews, two of which come from former CTEGD members:
- Trisha Dalapati & Julie M. Moore. Hemozoin: a Complex Molecule with Complex Activities. Curr Clin Micro Rpt 8, 87–102 (2021). https://doi.org/10.1007/s40588-021-00166-8 – Dalapati (BS 2019) is a former University of Georgia undergraduate who studied in Julie Moore’s laboratory. Dalapati is now at Duke University School of Medicine working on her M.D./Ph.D.
- Derek J. Pinto & Sumiti Vinayak. Cryptosporidium: Host-Parasite Interactions and Pathogenesis. Curr Clin Micro Rpt 8, 62–67 (2021). https://doi.org/10.1007/s40588-021-00159-7/ – Vinayak is a former CTEGD post-doctoral associate. She is now an assistant professor at the University of Illinois at Urbana-Champaign.
- Lucía Raquel Fernández, Daniel Musikant & Martin M. Edreira. Naturally Occurring Alkaloids, Derivatives, and Semi-synthetic Modifications as Lead Compounds for the Development of New Anti-Trypanosoma cruzi Agents. Curr Clin Micro Rpt 8, 68–86 (2021). https://doi.org/10.1007/s40588-021-00163-x
- Najara C. Bittencourt, Letícia P. Bertolla & Letusa Albrecht. Insights on Rosetting Phenomenon in Plasmodium vivax Malaria. Curr Clin Micro Rpt 8, 1–7 (2021). https://doi.org/10.1007/s40588-020-00155-3.