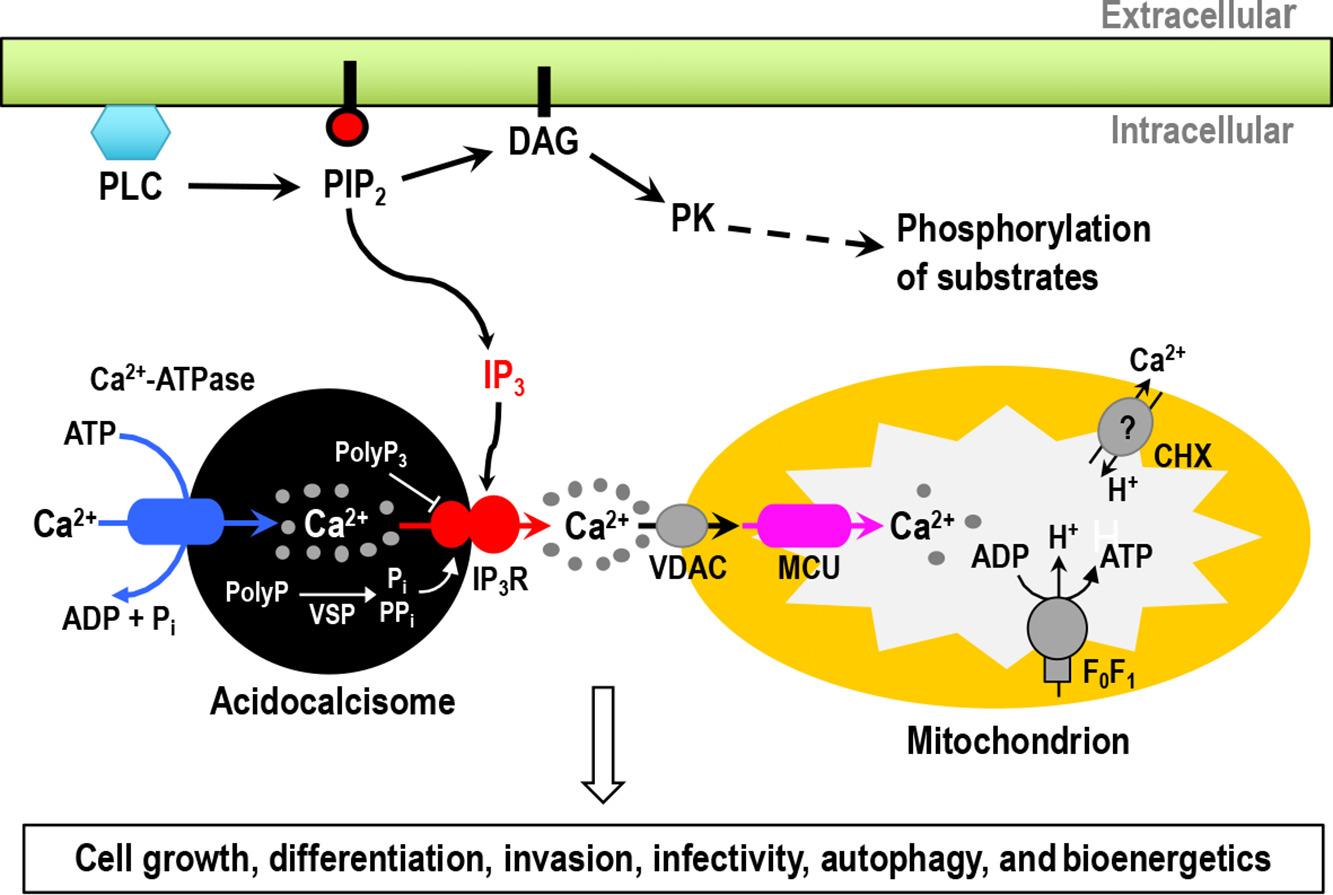
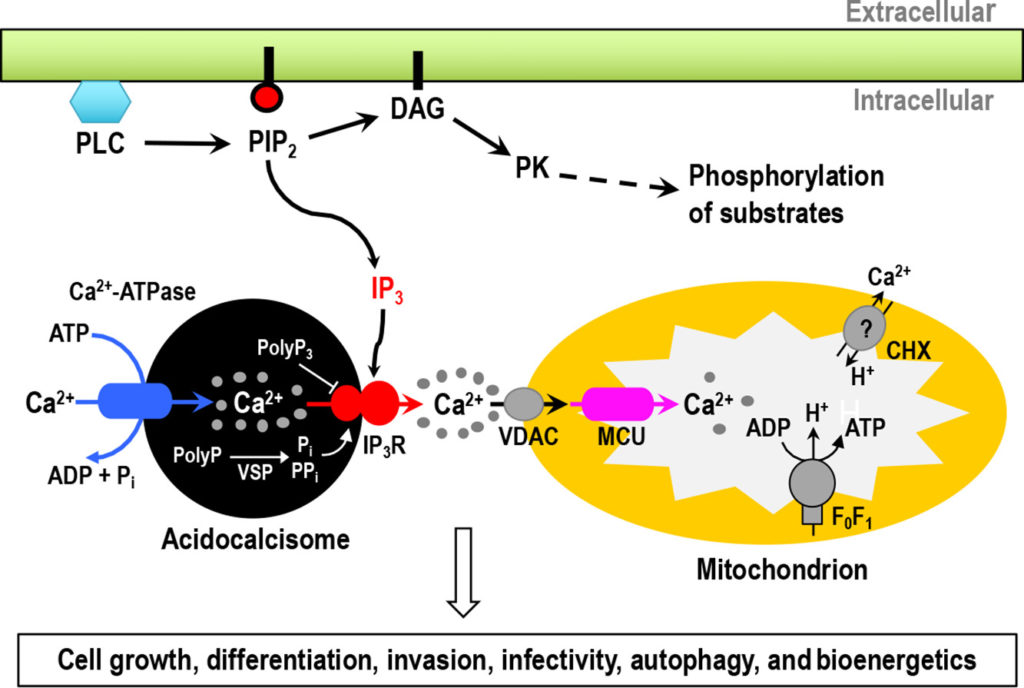
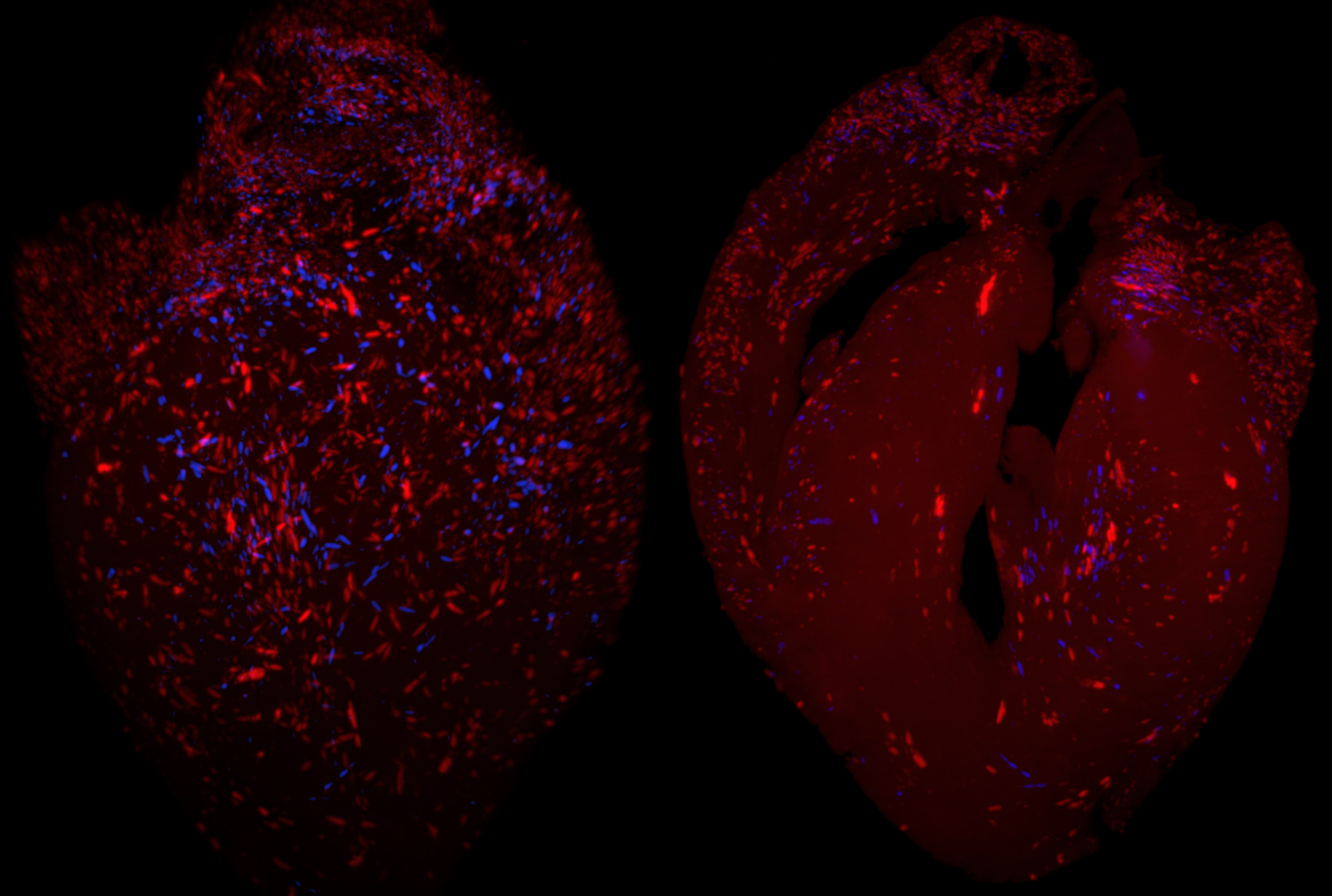
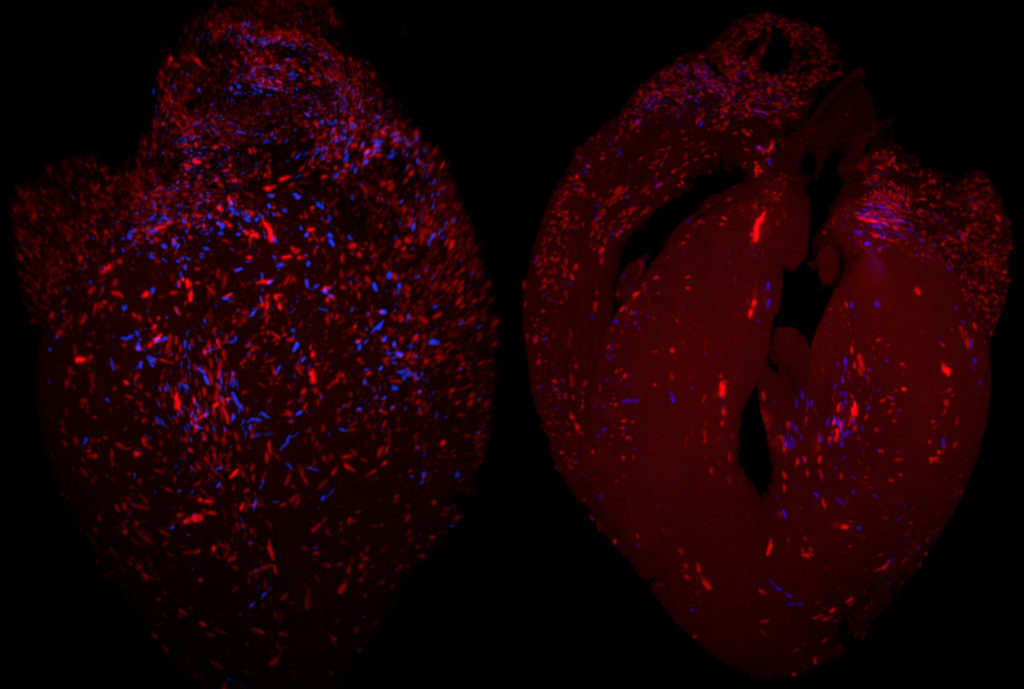
Reduced Trypanosoma cruzi-specific humoral response and enhanced T cell immunity after treatment interruption with benznidazole in chronic Chagas disease
Background: Interruption of benznidazole therapy due to the appearance of adverse effects, which is presumed to lead to treatment failure, is a major drawback in the treatment of chronic Chagas disease.
Methods: Trypanosoma cruzi-specific humoral and T cell responses, T cell phenotype and parasite load were measured to compare the outcome in 33 subjects with chronic Chagas disease treated with an incomplete benznidazole regimen and 58 subjects treated with the complete regimen, during a median follow-up period of 48 months.
Results: Both treatment regimens induced a reduction in the T. cruzi-specific antibody levels and similar rates of treatment failure when evaluated using quantitative PCR. Regardless of the regimen, polyfunctional CD4+ T cells increased in the subjects, with successful treatment outcome defined as a decrease of T. cruzi-specific antibodies. Regardless of the serological outcome, naive and central memory T cells increased after both regimens. A decrease in CD4+ HLA-DR+ T cells was associated with successful treatment in both regimens. The cytokine profiles of subjects with successful treatment showed fewer inflammatory mediators than those of the untreated T. cruzi-infected subjects. High levels of T cells expressing IL-7 receptor and low levels of CD8+ T cells expressing the programmed cell death protein 1 at baseline were associated with successful treatment following benznidazole interruption.
Conclusions: These findings challenge the notion that treatment failure is the sole potential outcome of an incomplete benznidazole regimen and support the need for further assessment of the treatment protocols for chronic Chagas disease.
Melisa D Castro Eiro, María A Natale, María G Alvarez, Huifeng Shen, Rodolfo Viotti, Bruno Lococo, Jacqueline Bua, Myriam Nuñez, Graciela L Bertocchi, María C Albareda, Gonzalo Cesar, Rick L Tarleton, Susana A Laucella. J Antimicrob Chemother. 2021 Mar 7;dkab054. doi: 10.1093/jac/dkab054