Diego Huet zeroes in on parasite that affects thousands each year
by Donna Huber

From an early age, Diego Huet has been interested in the unusual and fascinating found in the natural world.
His early encounters with animals, plants and insects nurtured his curiosity about nature. Their striking colors and sometimes strange shapes drew his interest, and even today he continues to capture them through macro photography. It was this fascination that led him to the parasite he studies today.
“I was always drawn to ‘unconventional’ or ‘weird’ science,” said Huet, an assistant professor in the College of Pharmacy’s Department of Pharmaceutical and Biomedical Sciences and member of the Center for Tropical and Emerging Global Diseases. “But I also wanted to be hands on, which is what led me to molecular and cellular biology.”
As a doctoral student, Huet began studying Trypanosoma brucei, a parasite commonly transmitted by the tsetse fly. Wanting to study a different parasite as a postdoctoral researcher, he was torn between studying Plasmodium, which is the causative agent of malaria, and Toxoplasma gondii, a related parasite that is carried by cats. Both parasites, which belong to a group of organisms called apicomplexans, cause diseases in humans and animals, and there remain large knowledge gaps in our basic understanding of them. Ultimately, he chose the latter.
“Plasmodium is difficult to manipulate,” Huet said. “Toxoplasma is related to Plasmodium, but is easier to work with because it isn’t as complex, and what we learn about Toxo could also increase our knowledge of Plasmodium.”
Just as yeast and fruit flies are used as model organisms to study human biology, Toxoplasma can be used as a model for shared features of apicomplexan biology.
Besides aiding in the understanding of other parasites of human and veterinary concern, including parasites that cause malaria in tropical and subtropical regions of the world, Toxoplasma gondii also causes human and animal disease. More than 40 million people in the U.S. are estimated to carry T. gondii. Although most never show symptoms, it poses a major health threat to immunocompromised individuals and pregnant women as it can lead to miscarriage and birth defects. Toxoplasmosis, the disease caused by Toxoplasma, is considered a leading cause of death among foodborne illnesses though it can also be transmitted through contact with cat feces.
The Centers for Disease Control and Prevention has it listed as a neglected parasitic infection in the United States and a target for public health action.
Huet joined the faculty at the University of Georgia in 2019 and has developed a robust research program to expand knowledge of the basic biology of Toxoplasma.

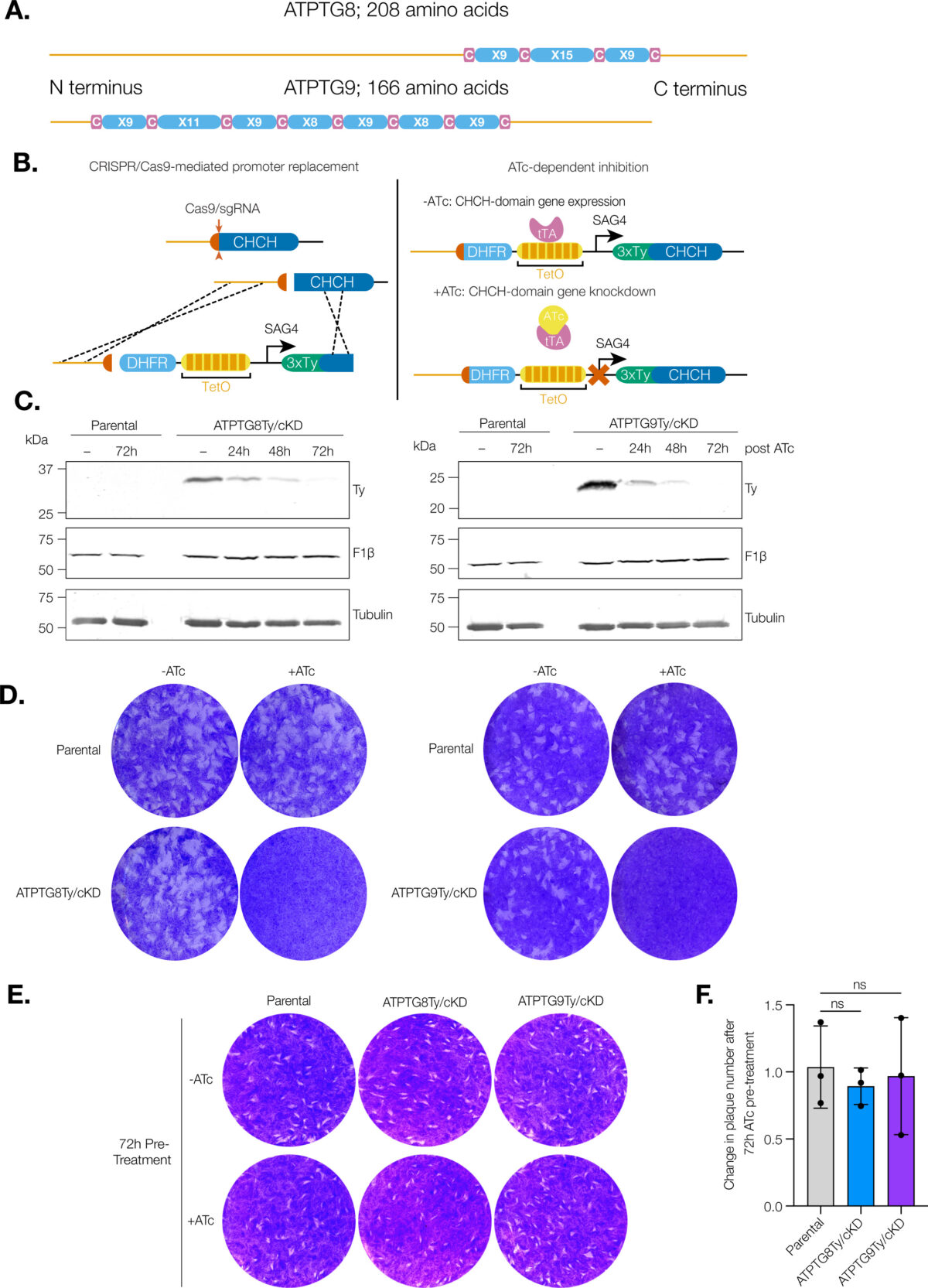
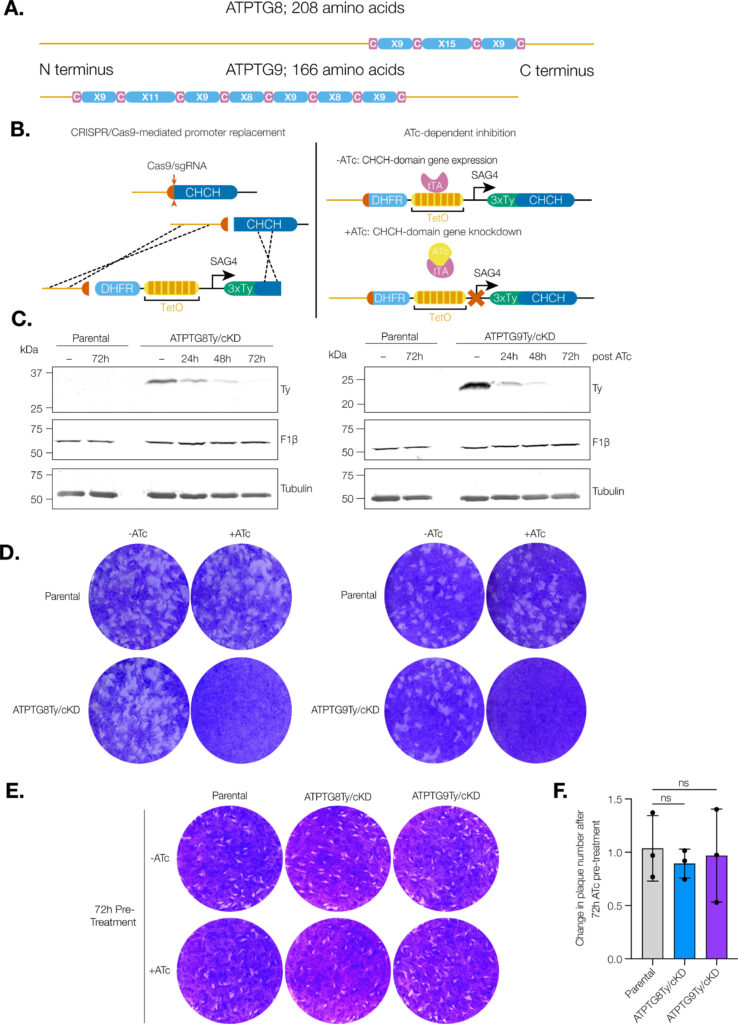
In a recently published study in “mBio”, cellular biology doctoral candidate and Huet Lab member Madelaine Usey looked at proteins critical for mitochondrial function in T. gondii. The mitochondrion is considered the “powerhouse of the cell,” but it is an enzyme called ATP synthase that generates the cellular energy.
“Our findings are really exciting for drug discovery,” Usey said. “Many of the proteins that make up the ATP synthase are different in Toxoplasma compared to other organisms. In this study, we were able to figure out what two of those novel subunits are doing—they act as scaffolding for this enormous ATP synthase complex.”
These proteins are unique to Toxoplasma and could be used in drug discovery as targets since they are important for mitochondrial functioning.
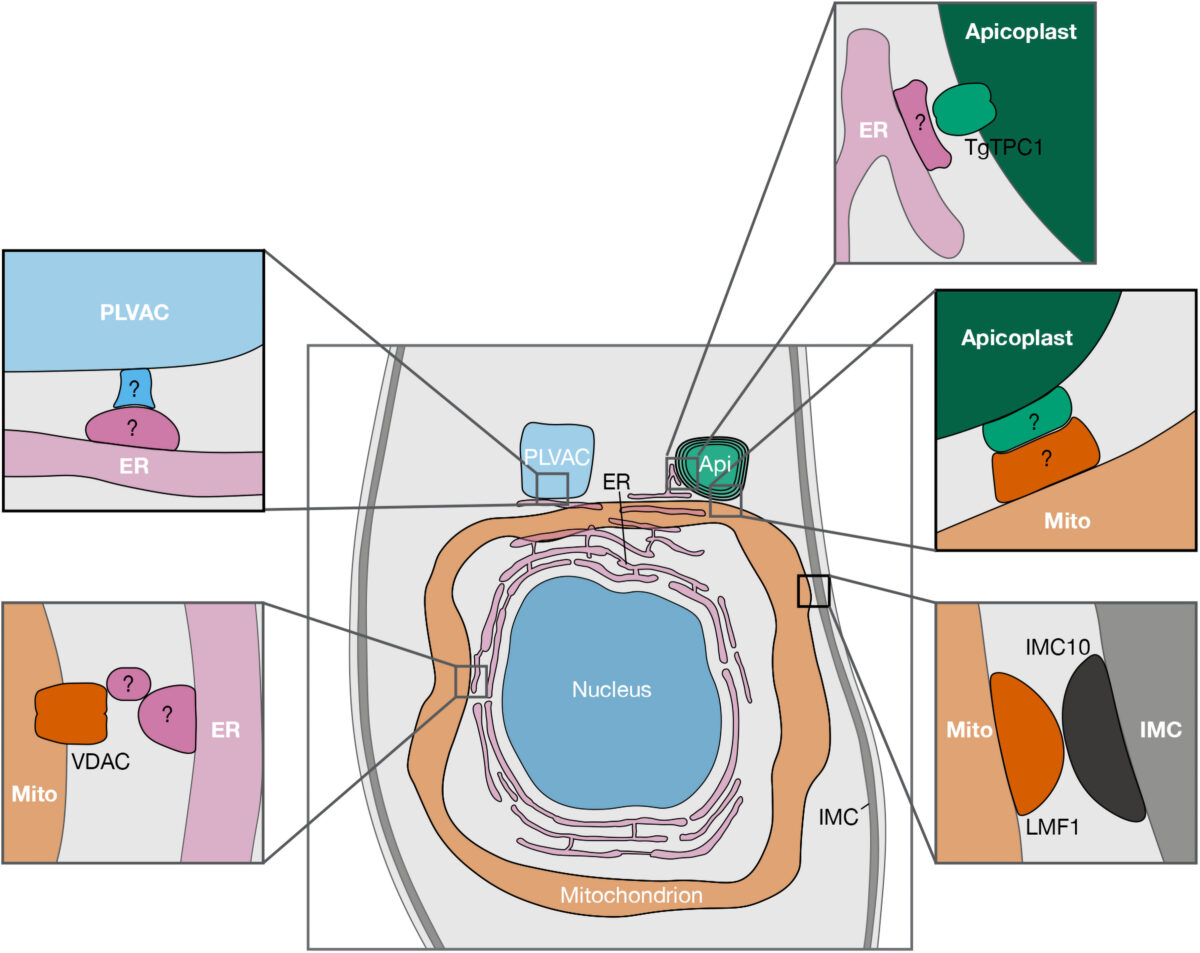
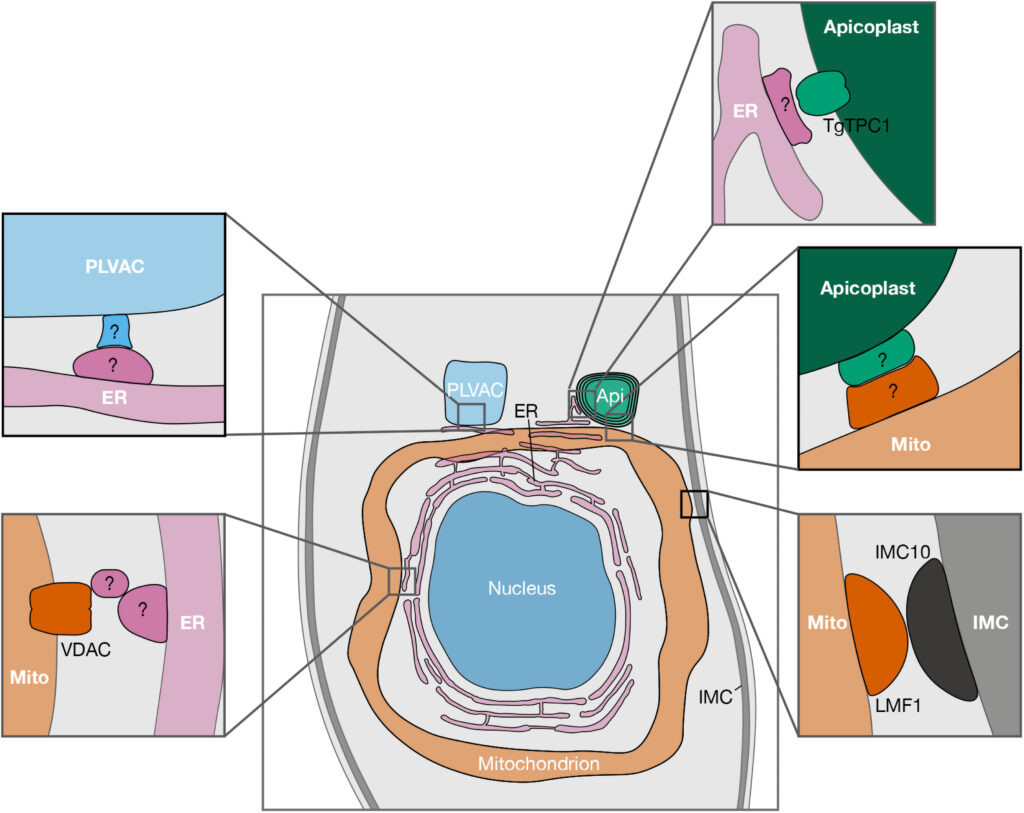
Another project in Huet’s laboratory, which recently received funding through a grant from the National Institute of General Medical Sciences, investigates how organelles within the parasite communicate.
“Traditionally, we thought organelles send and receive calcium and other metabolites in much the same way we receive a package through the mail,” Huet said. “Cells form vesicles to transport materials to specific locations within the cell. The vesicles are labeled with proteins that act like a postal address, telling the vesicle where to go.”
However, cells can also exchange material through another process.
“When the organelles’ membranes get close together, they form what is called a membrane contact site,” Huet said. “In this case it is more like one organelle hand delivers the package to another.”
A membrane contact site is a specialized protein structure that organelles use for intracellular communication. However, it is not a well understood structure in apicomplexans. In addition, these parasites have additional organelles not found in traditional models like humans and yeast, so Huet is trying to understand how the organellar communication is happening in apicomplexans using Toxoplasma as a model.
Identifying such proteins and their functions could lead to better drug targets and better drug treatments, which all the neglected parasitic diseases need.
“Toxo’s genome isn’t well annotated,” Huet said. “Finding membrane contact site proteins is an arduous task—it’s a goal of my lab to identify some of them and their involvement in Toxoplasma membrane contact sites.”
This article was first published at https://research.uga.edu/news/diego-huet-zeroes-in-on-parasite-that-affects-thousands-each-year/