Mitochondrial Pyruvate Carrier Subunits Are Essential for Pyruvate-Driven Respiration, Infectivity, and Intracellular Replication of Trypanosoma cruzi
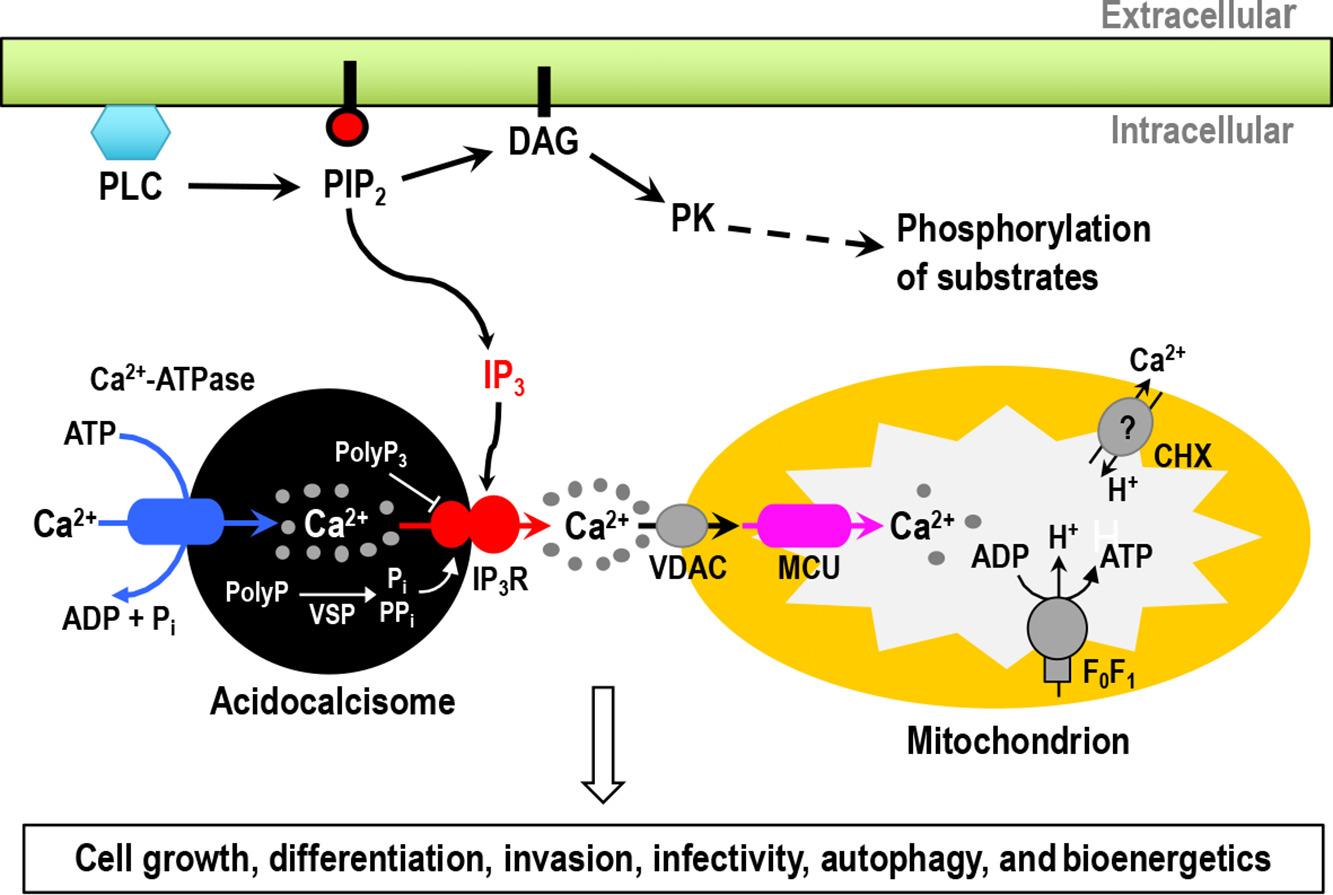
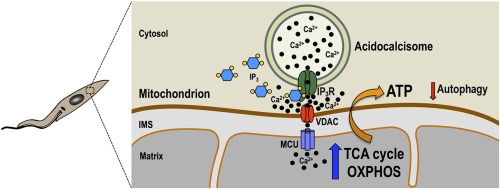
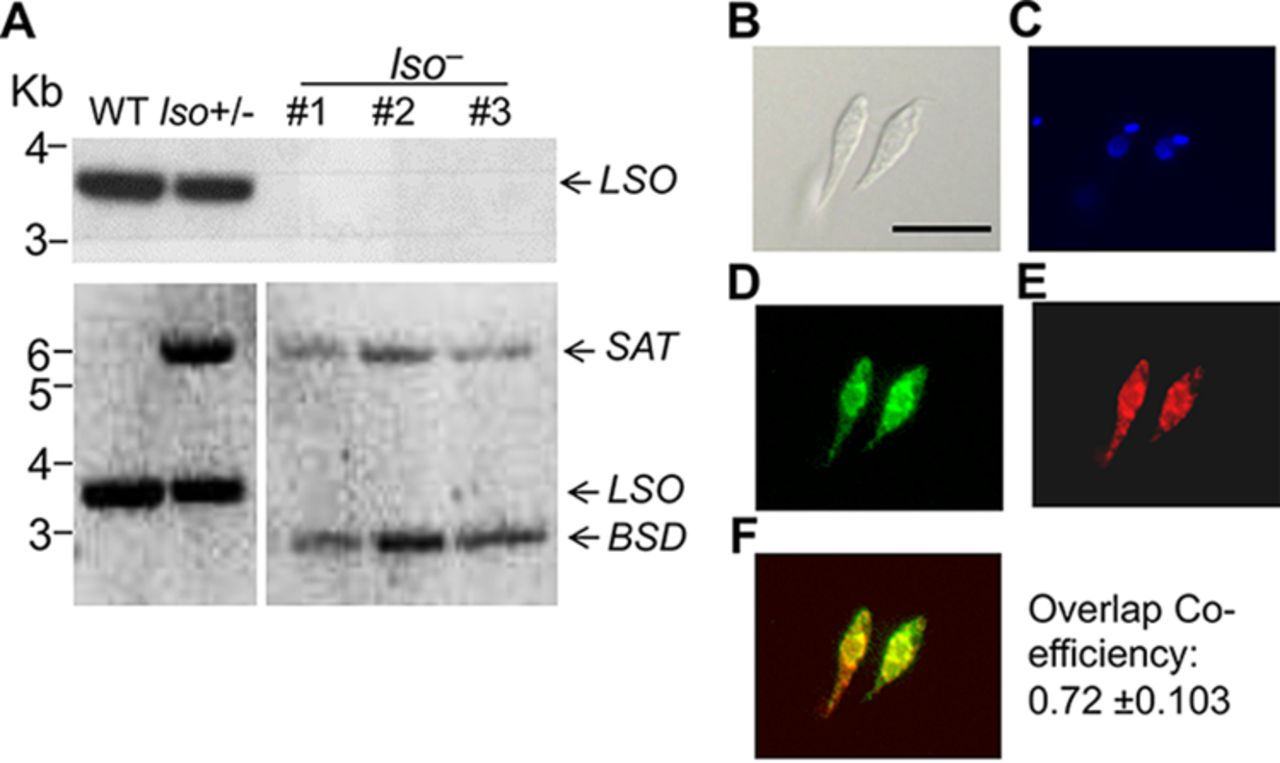
Pyruvate is the final metabolite of glycolysis and can be converted into acetyl coenzyme A (acetyl-CoA) in mitochondria, where it is used as the substrate for the tricarboxylic acid cycle. Pyruvate availability in mitochondria depends on its active transport through the heterocomplex formed by the mitochondrial pyruvate carriers 1 and 2 (MPC1/MPC2). We report here studies on MPC1/MPC2 of Trypanosoma cruzi, the etiologic agent of Chagas disease. Endogenous tagging of T. cruzi MPC1 (TcMPC1) and TcMPC2 with 3×c-Myc showed that both encoded proteins colocalize with MitoTracker to the mitochondria of epimastigotes. Individual knockout (KO) of TcMPC1 and TcMPC2 genes using CRISPR/Cas9 was confirmed by PCR and Southern blot analyses. Digitonin-permeabilized TcMPC1-KO and TcMPC2-KO epimastigotes showed reduced O2 consumption rates when pyruvate, but not succinate, was used as the mitochondrial substrate, while α-ketoglutarate increased their O2 consumption rates due to an increase in α-ketoglutarate dehydrogenase activity. Defective mitochondrial pyruvate import resulted in decreased Ca2+ uptake. The inhibitors UK5099 and malonate impaired pyruvate-driven oxygen consumption in permeabilized control cells. Inhibition of succinate dehydrogenase by malonate indicated that pyruvate needs to be converted into succinate to increase respiration. TcMPC1-KO and TcMPC2-KO epimastigotes showed little growth differences in standard or low-glucose culture medium. However, the ability of trypomastigotes to infect tissue culture cells and replicate as intracellular amastigotes was decreased in TcMPC-KOs. Overall, T. cruzi MPC1 and MPC2 are essential for cellular respiration in the presence of pyruvate, invasion of host cells, and replication of amastigotes.
IMPORTANCE Trypanosoma cruzi is the causative agent of Chagas disease. Pyruvate is the end product of glycolysis, and its transport into the mitochondrion is mediated by the mitochondrial pyruvate carrier (MPC) subunits. Using the CRISPR/Cas9 technique, we generated individual T. cruzi MPC1 (TcMPC1) and TcMPC2 knockouts and demonstrated that they are essential for pyruvate-driven respiration. Interestingly, although glycolysis was reported as not an important source of energy for the infective stages, MPC was essential for normal host cell invasion and intracellular replication.