Further insights of selenium-containing analogues of WC-9 against Trypanosoma cruzi
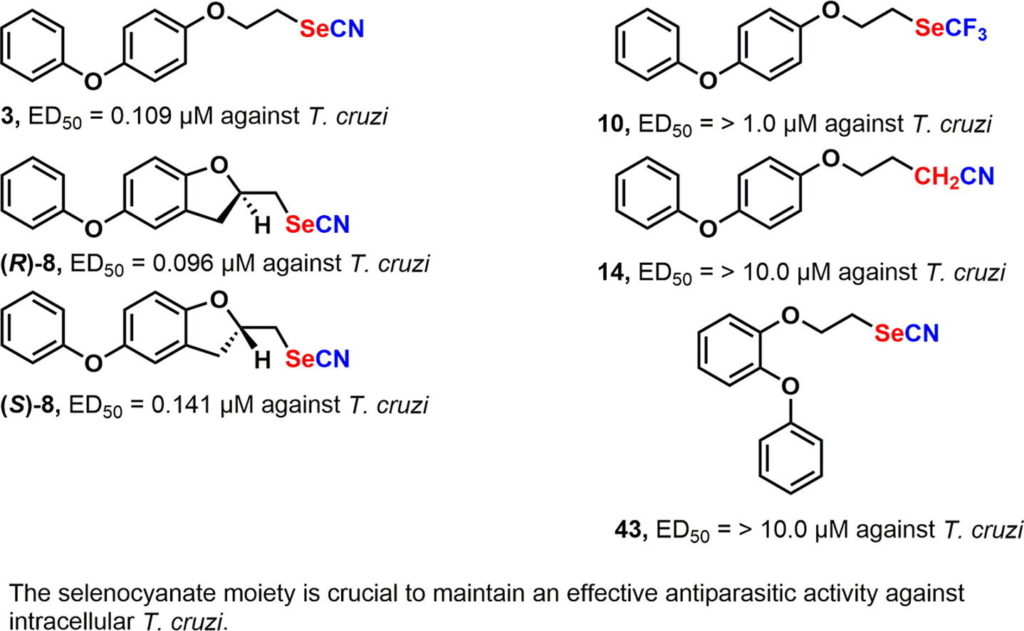
As a continuation of our project aimed at searching for new chemotherapeutic agents against American trypanosomiasis (Chagas disease), new selenocyanate derivatives were designed, synthesized and biologically evaluated against the clinically more relevant dividing form of Trypanosoma cruzi, the etiologic agent of this illness. In addition, in order to establish the role of each part of the selenocyanate moiety, different derivatives, in which the selenium atom or the cyano group were absent, were conceived, synthesized and biologically evaluated. In addition, in order to study the optimal position of the terminal phenoxy group, new regioisomers of WC-9 were synthesized and evaluated against T. cruzi. Finally, the resolution of a racemic mixture of a very potent conformationally rigid analogue of WC-9 was accomplished and further tested as growth inhibitors of T. cruzi proliferation. The results provide further insight into the role of the selenocyanate group in its antiparasitic activity.
María N. Chao, María V. Lorenzo-Ocampo, Sergio H. Szajnman, Roberto Docampo, Juan B. Rodriguez. 2019. Bioorganic & Medicinal Chemistry. https://doi.org/10.1016/j.bmc.2019.02.039
