Two CTEGD trainees receive AHA fellowships

Baihetiya “Barna” Baierna, a cellular biology graduate student in Silvia Moreno’s laboratory, received an American Heart Association Pre-doctoral Fellowship. It will fund her training for the next two years as she studies the mitochondrion of Toxoplasma gondii.
Baierna grew up wanting to follow in her mother’s footsteps as a scientist.
“My mom worked for the regional CDC in China and I was interested in science since a young age,” Baierna said.
After completing her undergraduate degree in biochemistry, she was sure she wanted to continue her training in graduate school. After being accepted into the Department of Cellular Biology program, she joined the Moreno Laboratory.
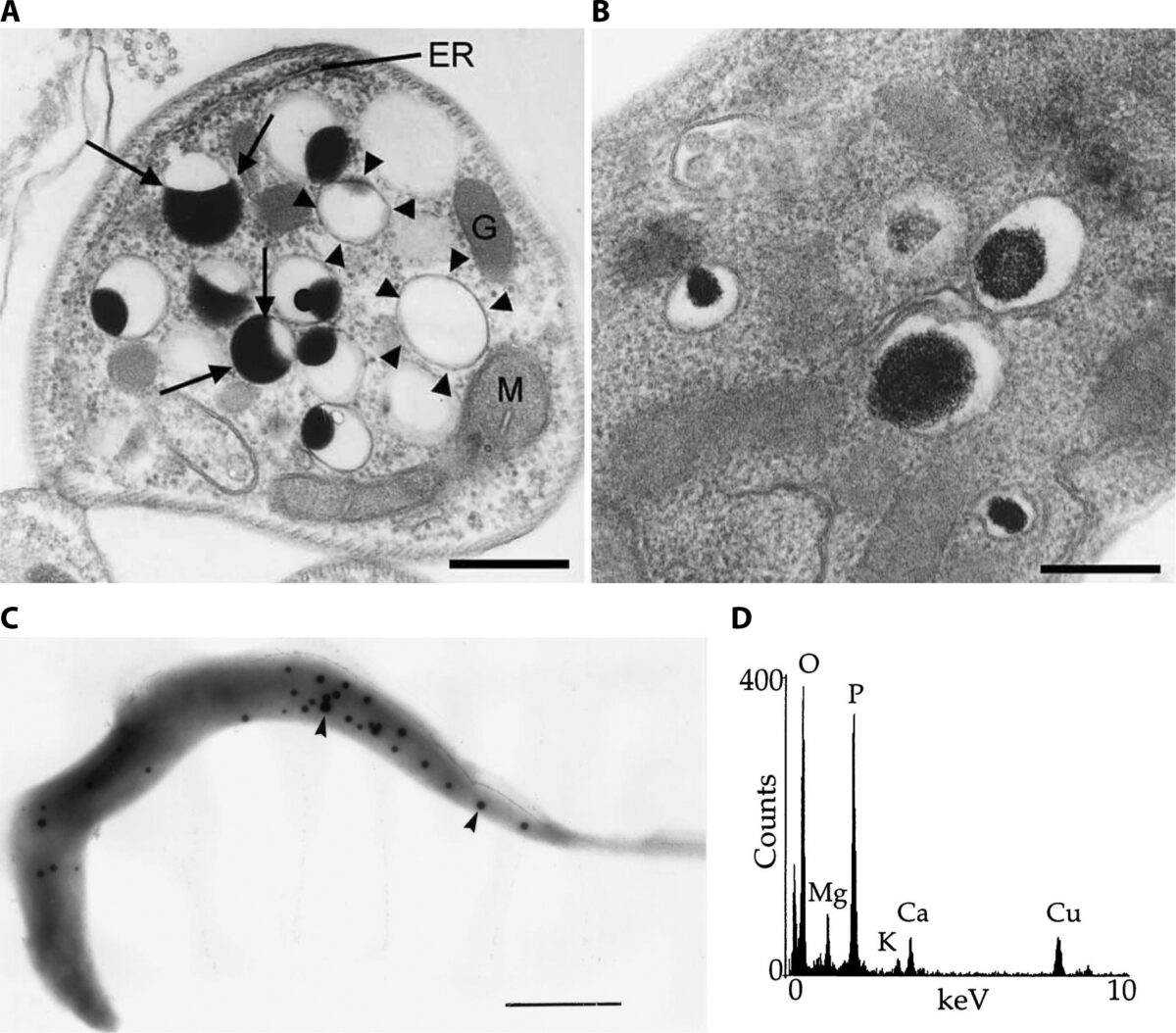
Toxoplasma gondii infects approximately one third of the world human population. The infection can cause serious complications in people with a suppressed immune system. Baierna’s research aims at validating novel T. gondii mitochondrial proteins as novel chemotherapeutic targets for improved chemotherapy of toxoplasmosis. This is important because the present drugs are not effective against the chronic stages of the infection. She has developed novel strategies for the discovery of new mitochondrial proteins and already found a novel enzymatic activity highly divergent from the mammalian counterpart. The outcome of this project will expand the knowledge of the T. gondii mitochondrion, as well as helping with the identification of viable drug targets.
“An AHA Fellowship is a very competitive award, but Barna deserves it and we are very proud of her,” said Moreno.
“Preparing the grant proposal was a great learning experience and it will help me with my career development,” said Baierna, “I’m very happy that it was funded.”
Mayara Bertolini, a post-doctoral fellow in Roberto Docampo’s laboratory, received an American Heart Association Post-doctoral Fellowship. It will support her training for one year.
After receiving her bachelor’s degree, Bertolini obtained her master’s degree in a lab that Docampo had set up in Brazil working on T. cruzi. From there she decided to pursue her Ph.D. at the University of Georgia. She completed her Ph.D. in 2023.
Trypanosoma cruzi is the parasite that causes Chagas disease. At least 6 million people, mostly in South America, are infected with the parasite. T. cruzi is transmitted to humans through the feces of an insect commonly referred to as the kissing bug. While Chagas disease was first discovered in 1909, there is still a lot that is unknown about the biology of T. cruzi. This lack of knowledge has hindered drug development. Bertolini’s project is focused on the role of polyphosphate during the Trypanosoma cruzi life cycle.
“This is the second fellowship from the AHA that Mayara has received. She got a two-year pre-doctoral fellowship before and has done outstanding work,” said Docampo.
“AHA Fellowships are very competitive and I’m thrilled my proposal was selected,” said Bertolini. “In addition to supporting my training, there is support for career development and networking opportunities.”
The story originally appeared at https://research.uga.edu/news/two-ctegd-trainees-receive-aha-fellowships/