Field Relevant Variation in Ambient Temperature Modifies Density-Dependent Establishment of Plasmodium falciparum Gametocytes in Mosquitoes
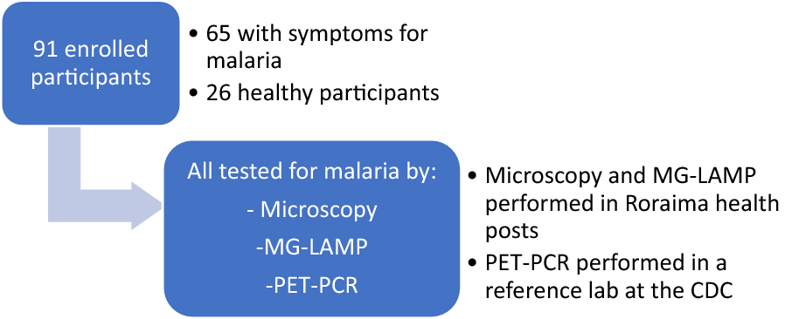
The relationship between Plasmodium falciparum gametocyte density and infections in mosquitoes is central to understanding the rates of transmission with important implications for control. Here, we determined whether field relevant variation in environmental temperature could also modulate this relationship. Anopheles stephensi were challenged with three densities of P. falciparum gametocytes spanning a ~10-fold gradient, and housed under diurnal/daily temperature range (“DTR”) of 9°C (+5°C and -4°C) around means of 20, 24, and 28°C. Vector competence was quantified as the proportion of mosquitoes infected with oocysts in the midguts (oocyst rates) or infectious with sporozoites in the salivary glands (sporozoite rates) at peak periods of infection for each temperature to account for the differences in development rates. In addition, oocyst intensities were also recorded from infected midguts and the overall study replicated across three separate parasite cultures and mosquito cohorts. While vector competence was similar at 20 DTR 9°C and 24 DTR 9°C, oocyst and sporozoite rates were also comparable, with evidence, surprisingly, for higher vector competence in mosquitoes challenged with intermediate gametocyte densities. For the same gametocyte densities however, severe reductions in the sporozoite rates was accompanied by a significant decline in overall vector competence at 28 DTR 9°C, with gametocyte density per se showing a positive and linear effect at this temperature. Unlike vector competence, oocyst intensities decreased with increasing temperatures with a predominantly positive and linear association with gametocyte density, especially at 28 DTR 9°C. Oocyst intensities across individual infected midguts suggested temperature-specific differences in mosquito susceptibility/resistance: at 20 DTR 9°C and 24 DTR 9°C, dispersion (aggregation) increased in a density-dependent manner but not at 28 DTR 9°C where the distributions were consistently random. Limitations notwithstanding, our results suggest that variation in temperature could modify seasonal dynamics of infectious reservoirs with implications for the design and deployment of transmission-blocking vaccines/drugs.
Ashutosh K. Pathak, Justine C. Shiau, Matthew B. Thomas and Courtney C. Murdock, Front Microbiol. 2019 Nov 15;10:2651. doi: 10.3389/fmicb.2019.02651. eCollection 2019.

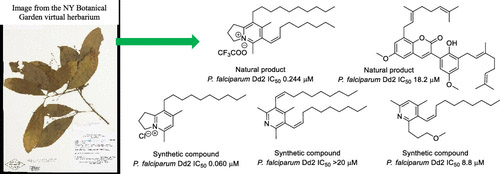
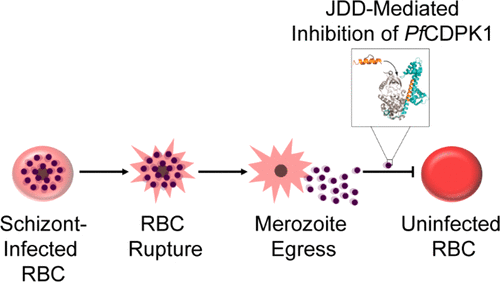
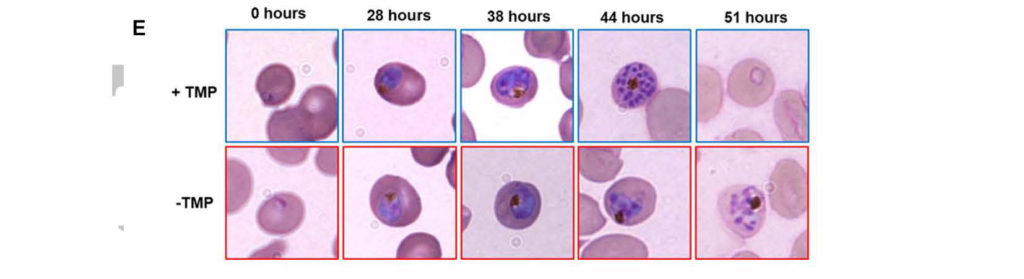 The vast majority of malaria mortality is attributed to one parasite species: Plasmodium falciparum. Asexual replication of the parasite within the red blood cell is responsible for the pathology of the disease. In Plasmodium, the endoplasmic reticulum (ER) is a central hub for protein folding and trafficking as well as stress response pathways. In this study, we tested the role of an uncharacterized ER protein, PfGRP170, in regulating these key functions by generating conditional mutants. Our data show that PfGRP170 localizes to the ER and is essential for asexual growth, specifically required for proper development of schizonts. PfGRP170 is essential for surviving heat shock, suggesting a critical role in cellular stress response. The data demonstrate that PfGRP170 interacts with the Plasmodium orthologue of the ER chaperone, BiP. Finally, we found that loss of PfGRP170 function leads to the activation of the Plasmodium eIF2α kinase, PK4, suggesting a specific role for this protein in this parasite stress response pathway.
The vast majority of malaria mortality is attributed to one parasite species: Plasmodium falciparum. Asexual replication of the parasite within the red blood cell is responsible for the pathology of the disease. In Plasmodium, the endoplasmic reticulum (ER) is a central hub for protein folding and trafficking as well as stress response pathways. In this study, we tested the role of an uncharacterized ER protein, PfGRP170, in regulating these key functions by generating conditional mutants. Our data show that PfGRP170 localizes to the ER and is essential for asexual growth, specifically required for proper development of schizonts. PfGRP170 is essential for surviving heat shock, suggesting a critical role in cellular stress response. The data demonstrate that PfGRP170 interacts with the Plasmodium orthologue of the ER chaperone, BiP. Finally, we found that loss of PfGRP170 function leads to the activation of the Plasmodium eIF2α kinase, PK4, suggesting a specific role for this protein in this parasite stress response pathway.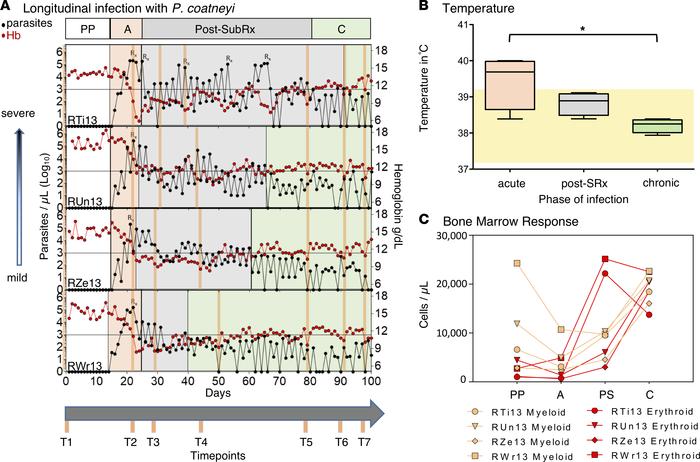 Chronic malaria is a major public health problem and significant challenge for disease eradication efforts. Despite its importance, the biological factors underpinning chronic malaria are not fully understood. Recent studies have shown that host metabolic state can influence malaria pathogenesis and transmission, but its role in chronicity is not known. Here, with the goal of identifying distinct modifications in the metabolite profiles of acute versus chronic malaria, metabolomics was performed on plasma from Plasmodium-infected humans and nonhuman primates with a range of parasitemias and clinical signs. In rhesus macaques infected with Plasmodium coatneyi, significant alterations in amines, carnitines, and lipids were detected during a high parasitemic acute phase and many of these reverted to baseline levels once a low parasitemic chronic phase was established. Plasmodium gene expression, studied in parallel in the macaques, revealed transcriptional changes in amine, fatty acid, lipid and energy metabolism genes, as well as variant antigen genes. Furthermore, a common set of amines, carnitines, and lipids distinguished acute from chronic malaria in plasma from human Plasmodium falciparum cases. In summary, distinct host-parasite metabolic environments have been uncovered that characterize acute versus chronic malaria, providing insights into the underlying host-parasite biology of malaria disease progression.
Chronic malaria is a major public health problem and significant challenge for disease eradication efforts. Despite its importance, the biological factors underpinning chronic malaria are not fully understood. Recent studies have shown that host metabolic state can influence malaria pathogenesis and transmission, but its role in chronicity is not known. Here, with the goal of identifying distinct modifications in the metabolite profiles of acute versus chronic malaria, metabolomics was performed on plasma from Plasmodium-infected humans and nonhuman primates with a range of parasitemias and clinical signs. In rhesus macaques infected with Plasmodium coatneyi, significant alterations in amines, carnitines, and lipids were detected during a high parasitemic acute phase and many of these reverted to baseline levels once a low parasitemic chronic phase was established. Plasmodium gene expression, studied in parallel in the macaques, revealed transcriptional changes in amine, fatty acid, lipid and energy metabolism genes, as well as variant antigen genes. Furthermore, a common set of amines, carnitines, and lipids distinguished acute from chronic malaria in plasma from human Plasmodium falciparum cases. In summary, distinct host-parasite metabolic environments have been uncovered that characterize acute versus chronic malaria, providing insights into the underlying host-parasite biology of malaria disease progression.