Dennis Kyle & CTEGD featured in Georgia Trend’s Article

Director Dennis Kyle was interviewed about CTEGD and his research as part of Georgia Trend’s feature article “Moving Innovation Beyond the Walls”. Read the article now.

Director Dennis Kyle was interviewed about CTEGD and his research as part of Georgia Trend’s feature article “Moving Innovation Beyond the Walls”. Read the article now.
by Amy Horton

New compound targets P. vivax, source of recent U.S. infections
Two University of Georgia researchers have been awarded approximately $770,000 from the Global Health Initiative Technology (GHIT) Fund to develop a new drug to kill the dormant liver stages of Plasmodium vivax, the most widespread of the malaria parasites. This amount is part of a total of JPY 334,238,778 awarded by the GHIT Fund to a partnership consisting of UGA, Medicine for Malaria Venture and Mitsubishi Tanabe Pharma Corporation.
“P. vivax often persists in the liver of patients, causing a relapse infection following treatment of the symptomatic blood infection,” said Steven Maher, associate research scientist in the Office of Research’s Center for Tropical and Emerging Global Diseases (CTEGD). “In many parts of the world, relapses account for the majority of total P. vivax cases.”
The announcement comes on the heels of reports of the first locally acquired cases of malaria in the United States in 20 years. In the summer of 2023, seven cases of locally acquired P. vivax malaria were reported in Sarasota, Fla., and one in Cameron County, Texas. These are in addition to a case of P. falciparum diagnosed in a Maryland resident living in the National Capital Region.
Most malaria cases diagnosed in the United States occur in people who have traveled to countries in South America, Africa, and southeast Asia where malaria is endemic. While locally acquired mosquito-transmitted malaria cases can occur, as Anopheles mosquito vectors exist throughout the United States, they are rare. The last reported outbreak was in 2003 when eight cases of locally acquired P. vivax malaria were identified in Palm Beach County, Fla.
The GHIT award will allow Maher and Chet Joyner to develop a compound series drug-screening program. Joyner is an assistant professor in the College of Veterinary Medicine’s Department of Infectious Diseases and Center for Vaccines and Immunology and jointly appointed to CTEGD.
The compound series identified by Maher, the result of testing more than 100,000 samples using infected liver cells, is the first new chemical class discovered in more than 70 years with efficacy against the persisting liver stage. Over the next two years, Maher and Joyner will be collaborating with Medicine for Malaria Venture and Mitsubishi Tanabe Pharma Corporation to alter the chemistry of the compound to improve drug-like properties, including half-life and potency, necessary to achieve single dose criteria.
“Discovering a drug to kill dormant, non-proliferating cells is extremely difficult, yet with the novel assay the team developed we now have the first new target and drug class with potential to accelerate global malaria elimination efforts,” said Dennis Kyle, director of the CTEGD.
The current drug class used to treat P. vivax malaria, 8-aminoquinolines, often results in serious side effects and cannot be administered to pregnant women, who are one of the patient groups most in need of treatment.
“We have the first validated compound that kills vivax while it lies dormant in the liver,” Joyner said. “We hope in the next two years to help advance the new compounds to clinical testing.”
Lisa K. Nolan, dean of the College of Veterinary Medicine, said the work Maher and Joyner are doing could deliver a better quality of life to millions of people around the world.
“This great research is a shining example of our commitment to translational research, which will take this drug from the lab to preclinical testing to the patient rapidly,” Nolan said.
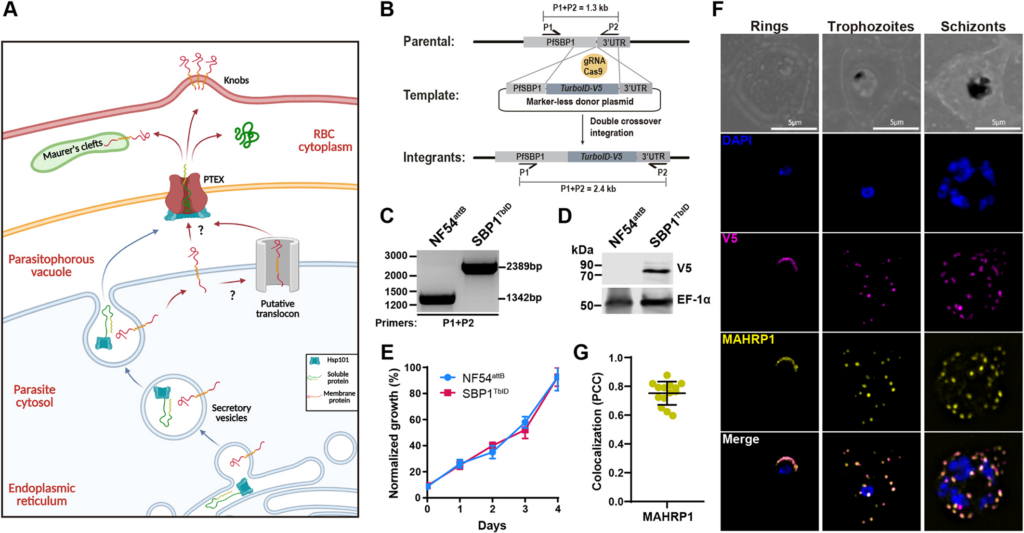
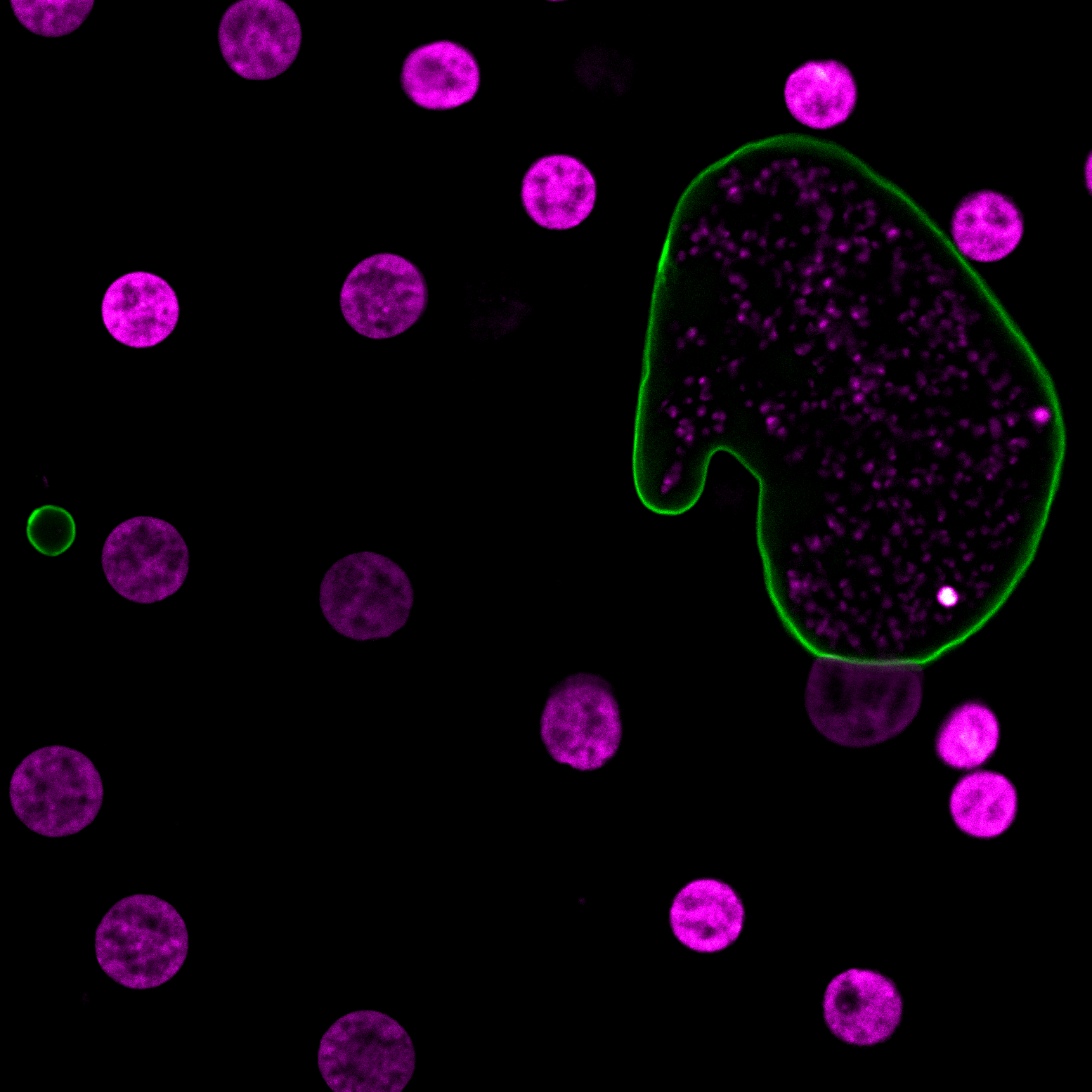
The malaria-causing parasite, Plasmodium falciparum completely remodels its host red blood cell (RBC) through the export of several hundred parasite proteins, including transmembrane proteins, across multiple membranes to the RBC. However, the process by which these exported membrane proteins are extracted from the parasite plasma membrane for export remains unknown. To address this question, we fused the exported membrane protein, skeleton binding protein 1 (SBP1), with TurboID, a rapid, efficient, and promiscuous biotin ligase (SBP1TbID). Using time-resolved, proximity biotinylation, and label-free quantitative proteomics, we identified two groups of SBP1TbID interactors: early interactors (pre-export) and late interactors (post-export). Notably, two promising membrane-associated proteins were identified as pre-export interactors, one of which possesses a predicted translocon domain, that could facilitate the export of membrane proteins. Further investigation using conditional mutants of these candidate proteins showed that these proteins were essential for asexual growth and localize to the host-parasite interface during early stages of the intraerythrocytic cycle. These data suggest that they may play a role in ushering membrane proteins from the PPM for export to the host RBC.
David Anaguano, Watcharatip Dedkhad, Carrie F Brooks, David W Cobb, Vasant Muralidharan. J Cell Sci. 2023 Sep 29;jcs.260506. doi: 10.1242/jcs.260506
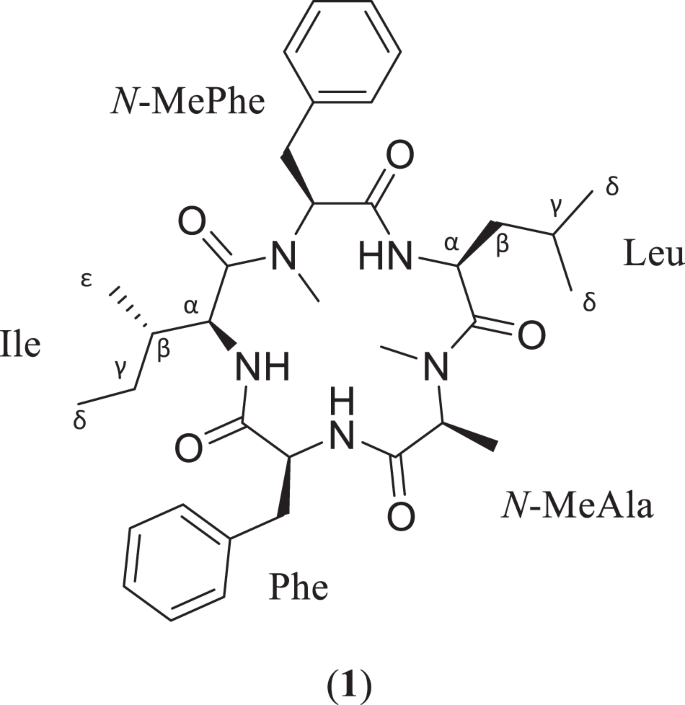
As part of ongoing efforts to isolate biologically active fungal metabolites, a cyclic pentapeptide, sheptide A (1), was discovered from strain MSX53339 (Herpotrichiellaceae). The structure and sequence of 1 were determined primarily by analysis of 2D NMR and HRMS/MS data, while the absolute configuration was assigned using a modified version of Marfey’s method. In an in vitro assay for antimalarial potency, 1 displayed a pEC50 value of 5.75 ± 0.49 against malaria-causing Plasmodium falciparum. Compound 1 was also tested in a counter screen for general cytotoxicity against human hepatocellular carcinoma (HepG2), yielding a pCC50 value of 5.01 ± 0.45 and indicating a selectivity factor of ~6. This makes 1 the third known cyclic pentapeptide biosynthesized by fungi with antimalarial activity.
Robert A Shepherd, Cody E Earp, Kristof B Cank, Huzefa A Raja, Joanna Burdette, Steven P Maher, Adriana A Marin, Anthony A Ruberto, Sarah Lee Mai, Blaise A Darveaux, Dennis E Kyle, Cedric J Pearce, Nicholas H Oberlies. J Antibiot (Tokyo). 2023 Sep 20. doi: 10.1038/s41429-023-00655-6.
Malaria is a deadly disease caused by the parasite Plasmodium and is transmitted through the bite of female Anopheles mosquitoes. The sporozoite stage of Plasmodium deposited by mosquitoes in the skin of vertebrate hosts undergoes a phase of mandatory development in the liver before initiating clinical malaria. We know little about the biology of Plasmodium development in the liver; access to the sporozoite stage and the ability to genetically modify such sporozoites are critical tools for studying the nature of Plasmodium infection and the resulting immune response in the liver. Here, we present a comprehensive protocol for the generation of transgenic Plasmodium berghei sporozoites. We genetically modify blood-stage P. berghei and use this form to infect Anopheles mosquitoes when they take a blood meal. After the transgenic parasites undergo development in the mosquitoes, we isolate the sporozoite stage of the parasite from the mosquito salivary glands for in vivo and in vitro experimentation. We demonstrate the validity of the protocol by generating sporozoites of a novel strain of P. berghei expressing the green fluorescent protein (GFP) subunit 11 (GFP11), and show how it could be used to investigate the biology of liver-stage malaria.
Carson Bowers, Samarchith P Kurup. J Vis Exp. 2023 May 5;(195). doi: 10.3791/64992.
It’s a microscopic parasite, invisible to the naked eye but common in tropical and subtropical regions throughout the world. Each year, millions of people are infected by Plasmodium and exposed to an even more debilitating—and often deadly—disease: malaria.
Malaria is one of the deadliest diseases known to man. It can lead to extreme illness, marked by fever, chills, headaches and fatigue. More than half the world’s population is at risk of contracting the disease, and those who develop relapsing infections suffer a host of associated costs.
Limited educational opportunities and wage loss lead to an often unbreakable cycle of poverty. Vulnerable populations are most at risk.
“When I’m teaching in an endemic area like Africa, it isn’t unusual to find a student who needs to sleep during part of the workshop because they have malaria,” researcher Jessica Kissinger said.
It’s a challenge she and her collaborators in the University of Georgia’s Center for Tropical and Emerging Global Diseases (CTEGD) are trying to combat.
When the Center was established in 1998, there were only a couple of faculty members studying Plasmodium. Now, 25 years later, it has become a world-class powerhouse of multidisciplinary malaria research. Scientists examine various species of the dangerous parasite, studying its life cycle and the mosquito that transmits it.
While Plasmodium seems to have superpowers that allow it to evade detection and resist treatment, CTEGD researchers are working together to innovate and transfer science from the lab to interventions on the ground.
Plasmodium is a complex organism, and studying it is like putting together a jigsaw puzzle. Some researchers contribute pieces related to the blood or liver stages of the parasite’s lifecycle, while others provide insights about hosts interactions. One way UGA’s research connects with the global effort to eradicate malaria is PlasmoDb—a resource derived in part from Kissinger’s research that is now part of a host of databases under the umbrella of The Eukaryotic Pathogen, Vector and Host information Resource (VEuPathDB).
“Our group has been able to help many others when their research question crosses into an –omic,” Kissinger said, referring to in-house shorthand for domains like genomics, proteomics and metabolomics.
Kissinger, Distinguished Research Professor of genetics in the Franklin College of Arts & Sciences, became interested in malaria and Plasmodium during her postdoctoral training at the National Institutes of Health (NIH). Working from an evolutionary biology perspective, she’s interested in how the parasite has changed over time.

“I see it as an arms race,” Kissinger said. “I want to understand what moves they have and can make.”
To understand the parasite, you must dive deep into its genetic code.
Kissinger paired her work in Plasmodium genomics with her interest in computing by helping create the database with information from the Plasmodium genome project completed in 2002. The Malaria Host-Pathogen Interaction Center, one of her projects at UGA, was a seven-year, multi-institutional effort funded, in part, by NIH to create data sets that could be used in systems biology of the host-pathogen interaction during the development of disease.
“Wouldn’t it be neat if, from the beginning of infection all the way to cure, you knew everything that was going on in the organism all the time?” Kissinger said, noting the project’s goal.
They generated terabytes of data that, along with data from the global research community, are publicly accessible and reusable through PlasmoDB and other resources.
Being part of a group that is studying so many different aspects of malaria helps put Kissinger’s research into perspective. Now, in addition to understanding the parasite, she also thinks about tools needed to facilitate research from peers.
David Peterson, professor of infectious diseases in the College of Veterinary Medicine, noted that low-tech solutions have mitigated malaria’s human costs. He acknowledged, however, that their long-term goals required more.
“We have to acknowledge that low-tech solutions, such as mosquito nets, have saved lives,” Peterson said. “But to develop the high-tech solutions that will one day end malaria, we need basic research.”
Pregnant women are particularly vulnerable to malaria because their existing immunity to malaria fails to protect them during pregnancy. Placental malaria often results in premature birth and low birth weight.
Peterson is interested in a binding protein that allows the parasite to adhere to the placenta. While many P. falciparum parasites have only one gene copy that encodes the placental binding protein, Peterson is investigating Plasmodiumisolates with two or more slightly different copies.
But why isn’t one copy enough?

That is the primary question Peterson is focused on. He wants to understand how Plasmodium uses extra copies to evade the immune system, distinguishing the role of each requires tools that Vasant Muralidharan, associate professor of cellular biology, has.
Muralidharan’s interest began when he contracted malaria himself. Through access to good health care, he made a full recovery, but the pain he endured remained. He wanted to understand this parasite. Even more, he wanted to make an impact with research.
His graduate training focused on biophysics, but soon his interest in Plasmodium resurfaced. He discovered there was a lack of tools to study the parasite on a genetic level.
“It’s like a house of cards, and each card is a gene,” Muralidharan said. “You can remove one and see what happens—does the house fall or remain standing?”
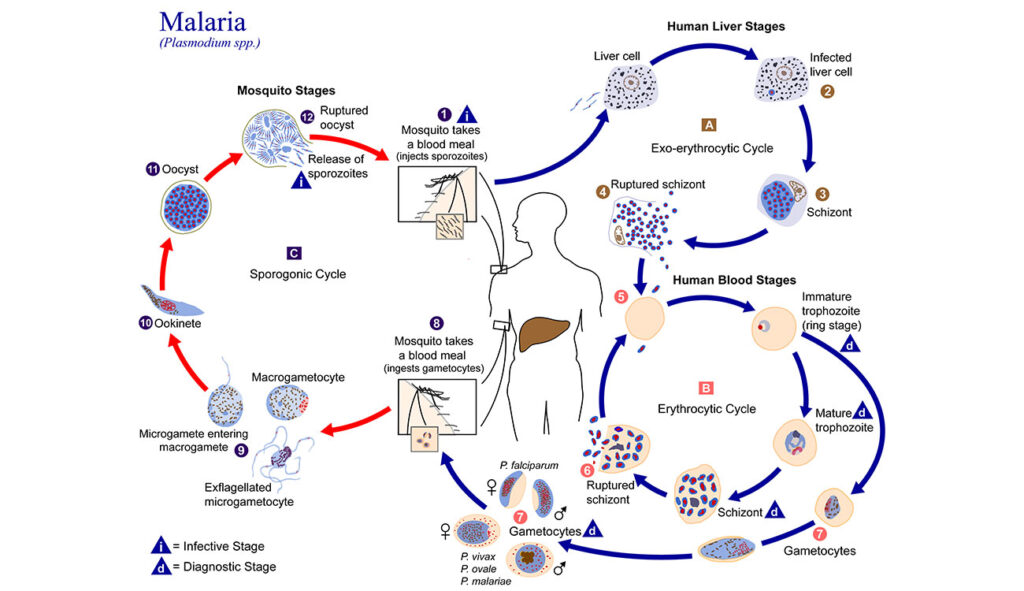
In the days before CRISPR/Cas9, there wasn’t a precise way to remove genes. Muralidharan is among the pioneers of gene-editing techniques in Plasmodium.
Like Peterson, Muralidharan focuses on proteins secreted by the parasite. He studies the largely unknown process that allows the parasite to invade a red blood cell (RBC), replicate and escape. The lack of tools was a major hindrance, so Muralidharan created new ones.
These tools have been used by Muralidharan’s CTEGD and CDC colleagues to see how drugs might fail. Muralidharan’s laboratory can create mutant Plasmodium parasites that become resistant to a particular drug, and genome sequence databases allow researchers to check if that mutant is already circulating in malaria endemic regions.

Plasmodium vivax is the predominant malaria parasite in Southeast Asia. It causes “relapsing malaria” during which some parasites go “dormant” after entering the liver instead of reproducing. This phase is a major obstacle for current treatments.
CTEGD Director Dennis Kyle, GRA Eminent Scholar Chair in Antiparasitic Drug Discovery and head of the Department of Cellular Biology, became fascinated with the Plasmodium parasite early in his career, spending time living in Thailand and working in refugee camps where malaria is prevalent.

“When I first got to the refugee camp and saw the situation people were living in, I questioned my decision to become a scientist in the lab instead of becoming a physician,” Kyle said, recalling a camp he worked in that housed about 1,300 kids between the ages of 2 and 15. “There was a guy who was a leader in the group who probably had no more than an early high school education. He said, ‘Look at what you can do—you might generate something that would benefit all of us. The physicians we have in the camp can only work on a few people at a time.’”
Kyle’s laboratory is looking to repurpose medications that have antimalarial properties, a safe way to reduce the development time from lab to clinical use. He’s optimistic we will see a drug treatment that eliminates vivax malaria.
“That’s where UGA is playing a major role,” he said. “The Gates Foundation funded us to develop tools to study the dormant parasite in the liver. And we’ve been successful.”
One of Kyle’s collaborators is Samarchith Kurup, assistant professor of cellular biology, who studies the human immune response to Plasmodium infection.
“We use mouse models to delve into the fundamental host-parasite interactions, which you cannot do practicallyin humans,” Kurup said. “Our understanding of these fundamental processes gives rise to newer and better vaccination approaches and drugs.”
Another important CTEGD addition is Chet Joyner, assistant professor of infectious diseases, whose work has helped make it easier to study dormant parasites stateside.
Like other Plasmodium researchers, Joyner became interested in parasites at an early age. During an undergraduate parasitology class, he discovered how little was known about P. vivax. He was already interested in how diseases develop, so for graduate school he focused on the liver stage of vivax malaria. However, it was a difficult task.


“At the time, the technologies weren’t there,” Joyner said. “Dennis was working on his system, but it wasn’t on the scene yet. I changed from studying the parasite to studying the animal model to understand pathogenesis and immunology in humans.”
Joyner joined UGA after completing his postdoctoral training at Emory University, where he developed a non-mouse animal model to study vivax malaria.
“We have to go to [Thailand] where people are infected and collect blood samples and then feed mosquitoes these samples to do the necessary studies,” Kyle said. “That’s been very impactful. We’ve gotten a lot of data out of it, and now with Chet’s model it all can be done under one roof.”
Joyner wants to understand the human immune response with a focus on vaccine development. Building on Muralidharan’s and other researchers’ findings of how the parasite interacts with the RBCs, Joyner’s vaccine program targets a specific protein in the parasite that inhibits the development of immunity.
“My colleagues have shown that if you knock this protein out in the parasite, the immune response in mice is actually great, and we are now working together to evaluate this in non-mouse models.” Joyner said.
Joyner also has collaborated with Belen Cassera, professor of biochemistry, to screen drug compounds. Cassera’s training focused on metabolism to find drug targets. She is particularly interested in how a drug functions.
“If we understand how the drug works, it will help us predict potential side effects in humans,” Cassera said. “We can’t predict everything, but knowing how it works gives you some confidence in whether it will work in humans.”
Cassera is focused on finding drugs that will treat the more lethal Plasmodium falciparum, the predominant species in Africa, which is rapidly becoming resistant to current treatments. Her work is complementary to Kyle’s.
“They run certain assays for the liver-stage infection, and our lab benefits because we want to know if the drug we are developing is specific for the blood stage or can tackle all stages,” Cassera said.

“Malaria is a vector-borne disease transmitted by a mosquito. You need to tackle not only the parasite in the human but also stop its transmission,” Cassera said. “CTEGD is unique because we can study the whole life cycle, including the mosquito.”
Michael Strand, H.M. Pulliam Chair of Entomology in the College of Agricultural and Environmental Sciences and a National Academy of Sciences Fellow, is an expert on parasite-host interactions. Instead of the human host, he is interested in mosquitoes. Recent work indicates blood feeding behavior of mosquitoes strongly affects malaria parasite development while the gut microbiota of mosquitos could lead to new ways to control populations. Having the SporoCore insectory on campus aids his research.
Established in 2020, SporoCore, under the management of Ash Pathak, assistant research scientist in the Department of Infectious Diseases, provides both uninfected and Plasmodium-infected Anopheles stephensi mosquitoes to researchers at UGA and other institutions. Like Joyner’s animal model, the insectory allows for research to be done in the U.S. that would otherwise require field work in an endemic country.
Old-school interventions like mosquito nets, combined with new drug therapies, have reduced the number of malaria deaths, which declined over the last 30 years before rising slightly during the COVID-19 pandemic. Great strides have been made to control and treat malaria—but not enough. New tools, like the ones being developed at CTEGD, are needed to keep pushing malaria’s morbidity and mortality rates in the right direction.
“The hard part—what can’t be done easily with the tools we already have—is being done,” Kyle said. “We just need new tools, which is one of the things that our center is really a leader in.”

Chet Joyner, PhD, a faculty member in the Center for Vaccines and Immunology and the Center for Tropical and Emerging Diseases in the College of Veterinary Medicine (CVM) at the University of Georgia, is the recipient of a $1.1 million grant from Open Philanthropy to perform preclinical testing of a vaccine designed to prevent reinfection from malaria.
“A vaccine that lessens the impact of this disease will have incalculable value in terms of lives saved and the quality of life of those in the affected areas,” said Lisa K. Nolan, DVM, PhD, dean of the CVM. “We are proud of Dr. Joyner’s work and that he has chosen to do it in the College of Veterinary Medicine at the University of Georgia.”
Joyner is collaborating with Dr. Richard Bucala, MD, PhD, of Yale University to test the vaccine that targets Plasmodium-encoded Macrophage Migration Inhibitory Factor (pMIF), a protein secreted by Plasmodium falciparum, a pathogen that causes malaria.
The science team for Open Philanthropy, which recommended grants to Joyner and Bucala for the three-year study, believes that vaccinating against pMIF may provide an important boost to the efficacy of existing malaria vaccines, according to a statement on its website, openphilanthropy.org.
Open Philanthropy is a Silicon Valley-based nonprofit which aims to use its resources to help others as much as possible. They fund work in many areas, including global health.
Joyner, who was recruited from Emory University to join the CVM in January of 2020, said the college is uniquely positioned to test the efficacy of the vaccine developed by Bucala at Yale.
“We are a strong malaria group with unique infrastructure and facilities that can support this necessary research within the CVM,” Joyner said.
Immunity to malaria is acquired naturally after exposure, but the disease can be fatal to children younger than five and debilitating up to age 10 because malaria parasites disrupt the immune system’s response with their own proteins that mimic the human Macrophage Migration Inhibitory Factor (MIF).
Not only does the resulting illness cause children to miss school, but it also leads to long-term cognitive decline due to nutritional deficiencies. Parents miss work to care for children and the economic impacts compound.
According to the World Health Organization’s 2022 World Malaria report, an estimated 247 million cases of malaria occurred worldwide in 2021 and 619,000 people died, mostly children under the age of five in sub-Saharan Africa.
Previous studies have suggested that a relationship exists between severity and transmissibility of malaria and variations in the gut microbiome, yet only limited information exists on the temporal dynamics of the gut microbial community during a malarial infection. Here, using a rhesus macaque model of relapsing malaria, we investigate how malaria affects the gut microbiome. In this study, we performed 16S sequencing on DNA isolated from rectal swabs of rhesus macaques over the course of an experimental malarial infection with Plasmodium cynomolgi and analyzed gut bacterial taxa abundance across primary and relapsing infections. We also performed metabolomics on blood plasma from the animals at the same timepoints and investigated changes in metabolic pathways over time. Members of Proteobacteria (family Helicobacteraceae) increased dramatically in relative abundance in the animal’s gut microbiome during peak infection while Firmicutes (family Lactobacillaceae and Ruminococcaceae), Bacteroidetes (family Prevotellaceae) and Spirochaetes amongst others decreased compared to baseline levels. Alpha diversity metrics indicated decreased microbiome diversity at the peak of parasitemia, followed by restoration of diversity post-treatment. Comparison with healthy subjects suggested that the rectal microbiome during acute malaria is enriched with commensal bacteria typically found in the healthy animal’s mucosa. Significant changes in the tryptophan-kynurenine immunomodulatory pathway were detected at peak infection with P. cynomolgi, a finding that has been described previously in the context of P. vivax infections in humans. During relapses, which have been shown to be associated with less inflammation and clinical severity, we observed minimal disruption to the gut microbiome, despite parasites being present. Altogether, these data suggest that the metabolic shift occurring during acute infection is associated with a concomitant shift in the gut microbiome, which is reversed post-treatment.
Danielle N Farinella, Sukhpreet Kaur, ViLinh Tran, Monica Cabrera-Mora, Chester J Joyner, Stacey A Lapp, Suman B Pakala, Mustafa V Nural, Jeremy D DeBarry, Jessica C Kissinger, Dean P Jones, Alberto Moreno, Mary R Galinski, Regina Joice Cordy. Front Cell Infect Microbiol. 2023 Jan 13;12:1058926. doi: 10.3389/fcimb.2022.1058926. eCollection 2022.
Malaria, caused by Plasmodium parasites is a severe disease affecting millions of people around the world. Plasmodium undergoes obligatory development and replication in the hepatocytes, before initiating the life-threatening blood-stage of malaria. Although the natural immune responses impeding Plasmodium infection and development in the liver are key to controlling clinical malaria and transmission, those remain relatively unknown. Here we demonstrate that the DNA of Plasmodium parasites is sensed by cytosolic AIM2 (absent in melanoma 2) receptors in the infected hepatocytes, resulting in Caspase-1 activation. Remarkably, Caspase-1 was observed to undergo unconventional proteolytic processing in hepatocytes, resulting in the activation of the membrane pore-forming protein, Gasdermin D, but not inflammasome-associated proinflammatory cytokines. Nevertheless, this resulted in the elimination of Plasmodium-infected hepatocytes and the control of malaria infection in the liver. Our study uncovers a pathway of natural immunity critical for the control of malaria in the liver.
Camila Marques-da-Silva, Barun Poudel, Rodrigo P Baptista, Kristen Peissig, Lisa S Hancox, Justine C Shiau, Lecia L Pewe, Melanie J Shears, Thirumala-Devi Kanneganti, Photini Sinnis, Dennis E Kyle, Prajwal Gurung, John T Harty, Samarchith P Kurup. Proc Natl Acad Sci U S A. 2023 Jan 10;120(2):e2210181120. doi: 10.1073/pnas.2210181120.
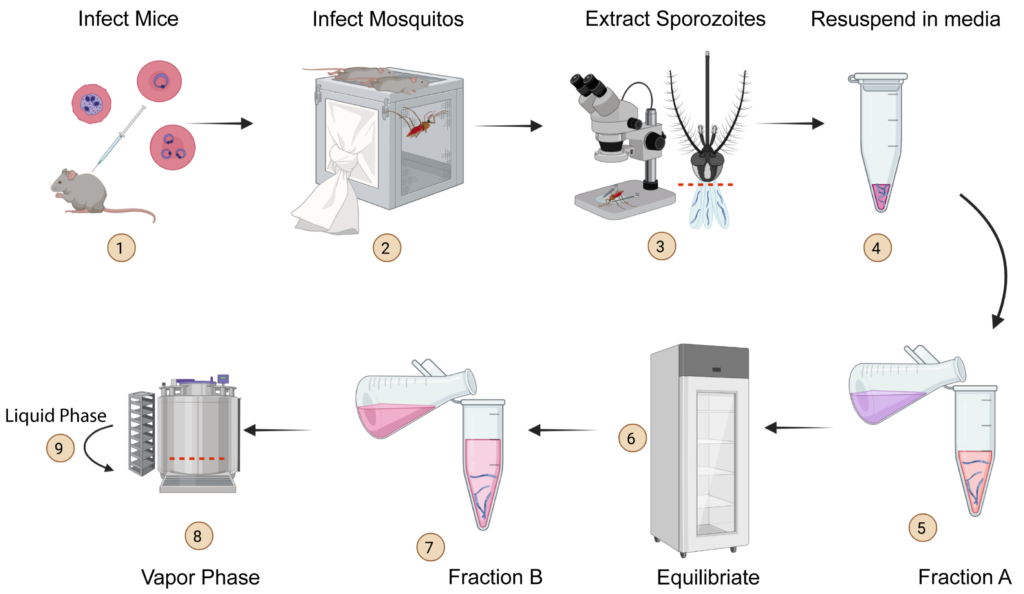
Malaria is a deadly disease caused by the parasite, Plasmodium, and impacts the lives of millions of people around the world. Following inoculation into mammalian hosts by infected mosquitoes, the sporozoite stage of Plasmodium undergoes obligate development in the liver before infecting erythrocytes and causing clinical malaria. The most promising vaccine candidates for malaria rely on the use of attenuated live sporozoites to induce protective immune responses. The scope of widespread testing or clinical use of such vaccines is limited by the absence of efficient, reliable, or transparent strategies for the long-term preservation of live sporozoites. Here we outline a method to cryopreserve the sporozoites of various human and murine Plasmodium species. We found that the structural integrity, viability, and in vivo or in vitro infectiousness were conserved in the recovered cryopreserved sporozoites. Cryopreservation using our approach also retained the transgenic properties of sporozoites and immunization with cryopreserved radiation attenuated sporozoites (RAS) elicited strong immune responses. Our work offers a reliable protocol for the long-term storage and recovery of human and murine Plasmodium sporozoites and lays the groundwork for the widespread use of live sporozoites for research and clinical applications.
Carson Bowers, Lisa Hancox, Kristen Peissig, Justine C. Shiau, Amélie Vantaux, Benoit Witkowski, Sivchheng Phal, Steven P. Maher, John T. Harty, Dennis E. Kyle, and Samarchith P. Kurup. Pathogens 2022, 11(12), 1487; https://doi.org/10.3390/pathogens11121487