Malaria and cryptosporidiosis, caused by apicomplexan parasites, remain major drivers of global child mortality. New drugs for the treatment of malaria and cryptosporidiosis, in particular, are of high priority; however, there are few chemically validated targets. The natural product cladosporin is active against blood- and liver-stage Plasmodium falciparum and Cryptosporidium parvum in cell-culture studies. Target deconvolution in P. falciparum has shown that cladosporin inhibits lysyl-tRNA synthetase (PfKRS1). Here, we report the identification of a series of selective inhibitors of apicomplexan KRSs. Following a biochemical screen, a small-molecule hit was identified and then optimized by using a structure-based approach, supported by structures of both PfKRS1 and C. parvum KRS (CpKRS). In vivo proof of concept was established in an SCID mouse model of malaria, after oral administration (ED90 = 1.5 mg/kg, once a day for 4 d). Furthermore, we successfully identified an opportunity for pathogen hopping based on the structural homology between PfKRS1 and CpKRS. This series of compounds inhibit CpKRS and C. parvum and Cryptosporidium hominis in culture, and our lead compound shows oral efficacy in two cryptosporidiosis mouse models. X-ray crystallography and molecular dynamics simulations have provided a model to rationalize the selectivity of our compounds for PfKRS1 and CpKRS vs. (human) HsKRS. Our work validates apicomplexan KRSs as promising targets for the development of drugs for malaria and cryptosporidiosis.
Beatriz Baragaña, Barbara Forte, Ryan Choi, Stephen Nakazawa Hewitt, Juan A. Bueren-Calabuig, João Pedro Pisco, Caroline Peet, David M. Dranow, David A. Robinson, Chimed Jansen, Neil R. Norcross, Sumiti Vinayak, Mark Anderson, Carrie F. Brooks, Caitlin A. Cooper, Sebastian Damerow, Michael Delves, Karen Dowers, James Duffy, Thomas E. Edwards, Irene Hallyburton, Benjamin G. Horst, Matthew A. Hulverson, Liam Ferguson, María Belén Jiménez-Díaz, Rajiv S. Jumani, Donald D. Lorimer, Melissa S. Love, Steven Maher, Holly Matthews, Case W. McNamara, Peter Miller, Sandra O’Neill, Kayode K. Ojo, Maria Osuna-Cabello, Erika Pinto, John Post, Jennifer Riley, Matthias Rottmann, Laura M. Sanz, Paul Scullion, Arvind Sharma, Sharon M. Shepherd, Yoko Shishikura, Frederick R. C. Simeons, Erin E. Stebbins, Laste Stojanovski, Ursula Straschil, Fabio K. Tamaki, Jevgenia Tamjar, Leah S. Torrie, Amélie Vantaux, Benoît Witkowski, Sergio Wittlin, Manickam Yogavel, Fabio Zuccotto, Iñigo Angulo-Barturen, Robert Sinden, Jake Baum, Francisco-Javier Gamo, Pascal Mäser, Dennis E. Kyle, Elizabeth A. Winzeler, Peter J. Myler, Paul G. Wyatt, David Floyd, David Matthews, Amit Sharma, Boris Striepen, Christopher D. Huston, David W. Gray, Alan H. Fairlamb, Andrei V. Pisliakov, Chris Walpole, Kevin D. Read, Wesley C. Van Voorhis, and Ian H. Gilbert. 2019. PNAS, https://doi.org/10.1073/pnas.1814685116

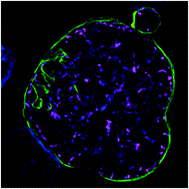 Advanced cell culture methods for modeling organ-level structure have been demonstrated to replicate in vivo conditions more accurately than traditional in vitro cell culture. Given that the liver is particularly important to human health, several advanced culture methods have been developed to experiment with liver disease states, including infection with Plasmodium parasites, the causative agent of malaria. These models have demonstrated that intrahepatic parasites require functionally stable hepatocytes to thrive and robust characterization of the parasite populations’ response to investigational therapies is dependent on high-content and high-resolution imaging (HC/RI). We previously reported abiotic confinement extends the functional longevity of primary hepatocytes in a microfluidic platform and set out to instill confinement in a microtiter plate platform while maintaining optical accessibility for HC/RI; with an end-goal of producing an improved P. vivax liver stage culture model. We developed a novel fabrication process in which a PDMS soft mold embosses hepatocyte-confining microfeatures into polystyrene, resulting in microfeature-based hepatocyte confinement (μHEP) slides and plates. Our process was optimized to form both microfeatures and culture wells in a single embossing step, resulting in a 100 μm-thick bottom ideal for HC/RI, and was found inexpensively amendable to microfeature design changes. Microfeatures improved intrahepatic parasite infection rates and μHEP systems were used to reconfirm the activity of reference antimalarials in phenotypic dose-response assays. RNAseq of hepatocytes in μHEP systems demonstrated microfeatures sustain hepatic differentiation and function, suggesting broader utility for preclinical hepatic assays; while our tailorable embossing process could be repurposed for developing additional organ models.
Advanced cell culture methods for modeling organ-level structure have been demonstrated to replicate in vivo conditions more accurately than traditional in vitro cell culture. Given that the liver is particularly important to human health, several advanced culture methods have been developed to experiment with liver disease states, including infection with Plasmodium parasites, the causative agent of malaria. These models have demonstrated that intrahepatic parasites require functionally stable hepatocytes to thrive and robust characterization of the parasite populations’ response to investigational therapies is dependent on high-content and high-resolution imaging (HC/RI). We previously reported abiotic confinement extends the functional longevity of primary hepatocytes in a microfluidic platform and set out to instill confinement in a microtiter plate platform while maintaining optical accessibility for HC/RI; with an end-goal of producing an improved P. vivax liver stage culture model. We developed a novel fabrication process in which a PDMS soft mold embosses hepatocyte-confining microfeatures into polystyrene, resulting in microfeature-based hepatocyte confinement (μHEP) slides and plates. Our process was optimized to form both microfeatures and culture wells in a single embossing step, resulting in a 100 μm-thick bottom ideal for HC/RI, and was found inexpensively amendable to microfeature design changes. Microfeatures improved intrahepatic parasite infection rates and μHEP systems were used to reconfirm the activity of reference antimalarials in phenotypic dose-response assays. RNAseq of hepatocytes in μHEP systems demonstrated microfeatures sustain hepatic differentiation and function, suggesting broader utility for preclinical hepatic assays; while our tailorable embossing process could be repurposed for developing additional organ models.