First evidence of polychaete intermediate hosts for Neospirorchis spp. marine turtle blood flukes (Trematoda: Spirorchiidae)
Abstract
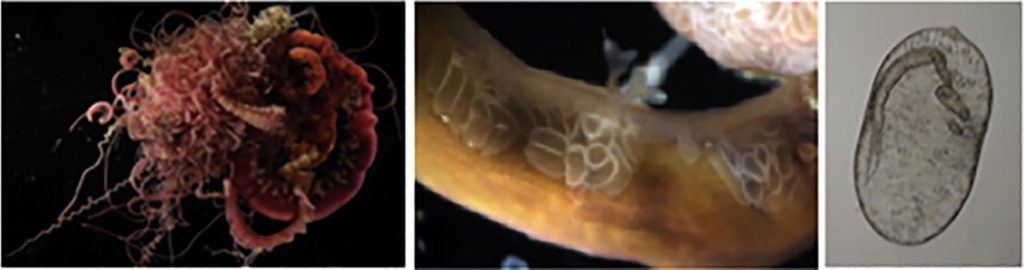
Life cycles of spirorchiids that infect the vascular system of turtles are poorly understood. Few life cycles of these blood flukes have been elucidated and all intermediate hosts reported are gastropods (Mollusca), regardless of whether the definitive host is a freshwater or a marine turtle. During a recent survey of blood fluke larvae in polychaetes on the coast of South Carolina, USA, spirorchiid-like cercariae were found to infect the polychaetes Amphitrite ornata (Terebellidae) and Enoplobranchus sanguineus (Polycirridae). Cercariae were large, furcate, with a ventral acetabulum, but no eyespots were observed. Partial sequences of D1–D2 domains of the large ribosomal subunit, the internal transcribed spacer 2, and the mitochondrial cytochrome oxidase 1 genes allowed the identification of sporocysts and cercariae as belonging to two unidentified Neospirorchis species reported from the green turtle, Chelonia mydas, in Florida: Neospirorchis sp. (Neogen 13) in A. ornata and Neospirorchis sp. (Neogen 14) in E. sanguineus. Phylogenetic analysis suggests that infection of annelids by blood flukes evolved separately in aporocotylids and spirorchiids. Our results support the contention that the Spirorchiidae is not a valid family and suggest that Neospirorchis is a monophyletic clade within the paraphyletic Spirorchiidae. Since specificity of spirorchiids for their intermediate hosts is broader than it was thus far assumed, surveys of annelids in turtle habitats are necessary to further our understanding of the life history of these pathogenic parasites.
Isaure de Buron, Beatrice L. Colon, Sasha V. Siegel, Jenna Oberstaller, Andrea Rivero, Dennis E. Kyle. 2018. International Journal for Parasitology; 48(14):1097-1106. https://doi.org/10.1016/j.ijpara.2018.08.002

