Two CTEGD faculty members receive Creative Research Awards
Jessica Kissinger and Dennis Kyle received the Lamar Dodd Creative Research Award during UGA’s Honors Week. The award recognizes established investigators whose overall scholarly body of work has had a major impact on the field of study and has established the investigator’s international reputation as a leader in the field.
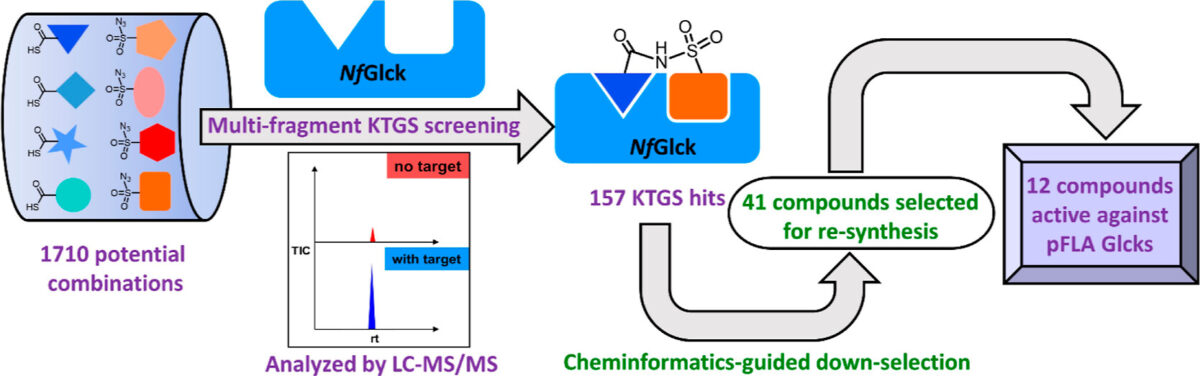
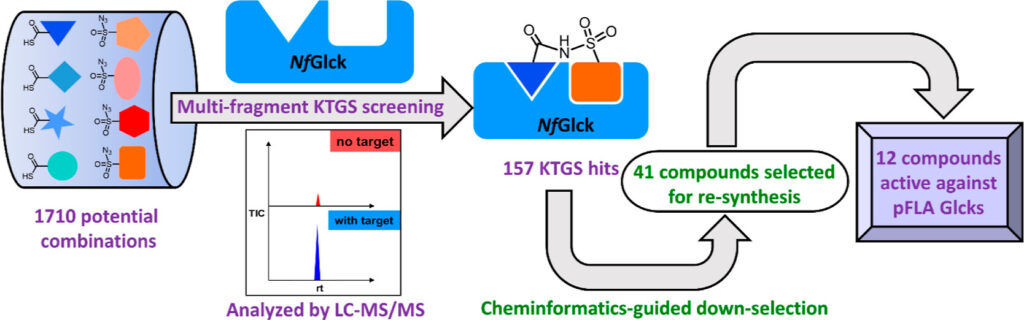
Jessica Kissinger, Distinguished Research Professor in the Franklin College of Arts and Sciences’ genetics department and former director of the UGA Institute of Bioinformatics, has focused her interdisciplinary career on the question of how parasites evolve. She has been a driving force behind the groundbreaking effort to create and maintain novel bioinformatics databases covering omics data for hundreds of dangerous pathogens. The Eukaryotic Pathogen, Vector, and Host Informatics Resources knowledgebase (VEuPathDB.org) is an integrated, centralized resource for data mining on more than 500 organisms. Databases searches are free, permitting researchers to gain insights into and test hypotheses that may pave the way for new approaches to treating or preventing diseases such as malaria and Cryptosporidium (a waterborne parasite). Kissinger has used the databases and other bioinformatics tools to make remarkable discoveries.
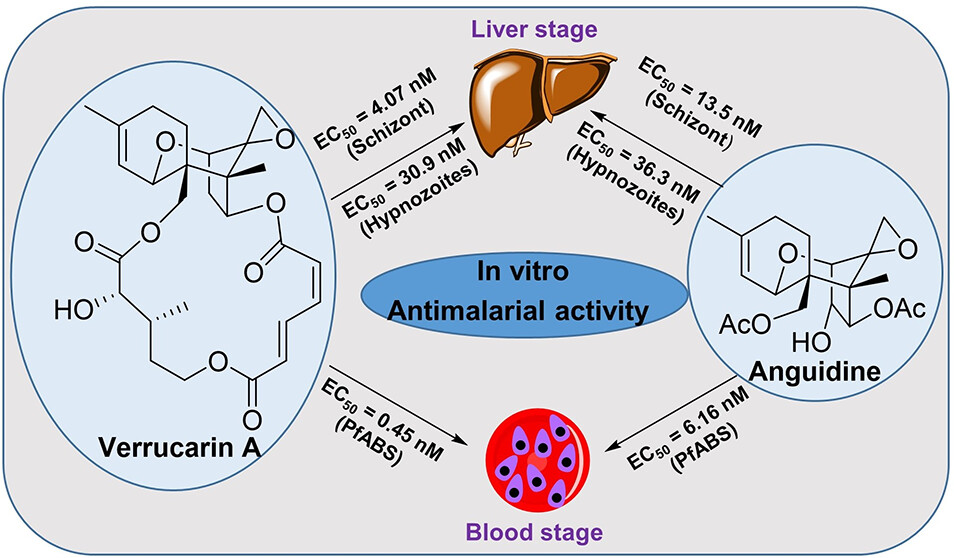
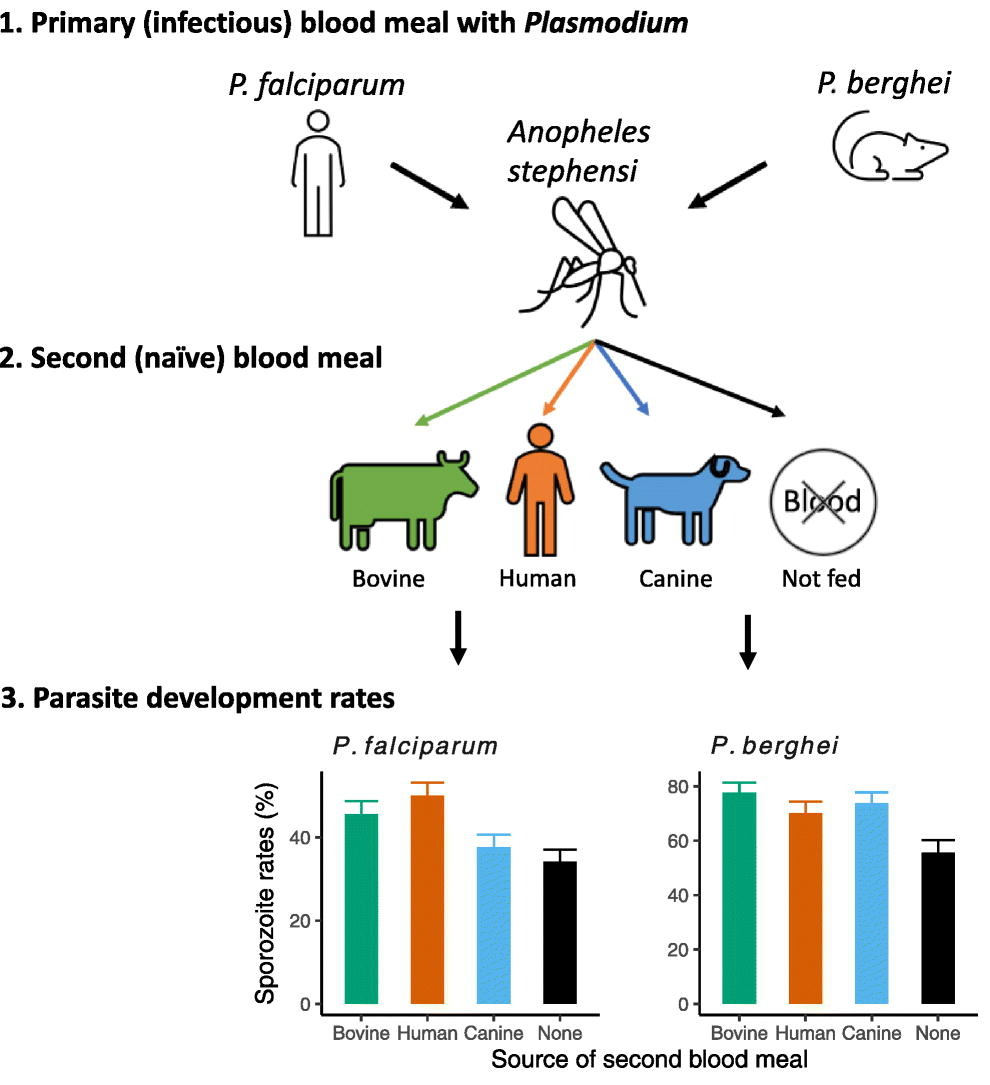
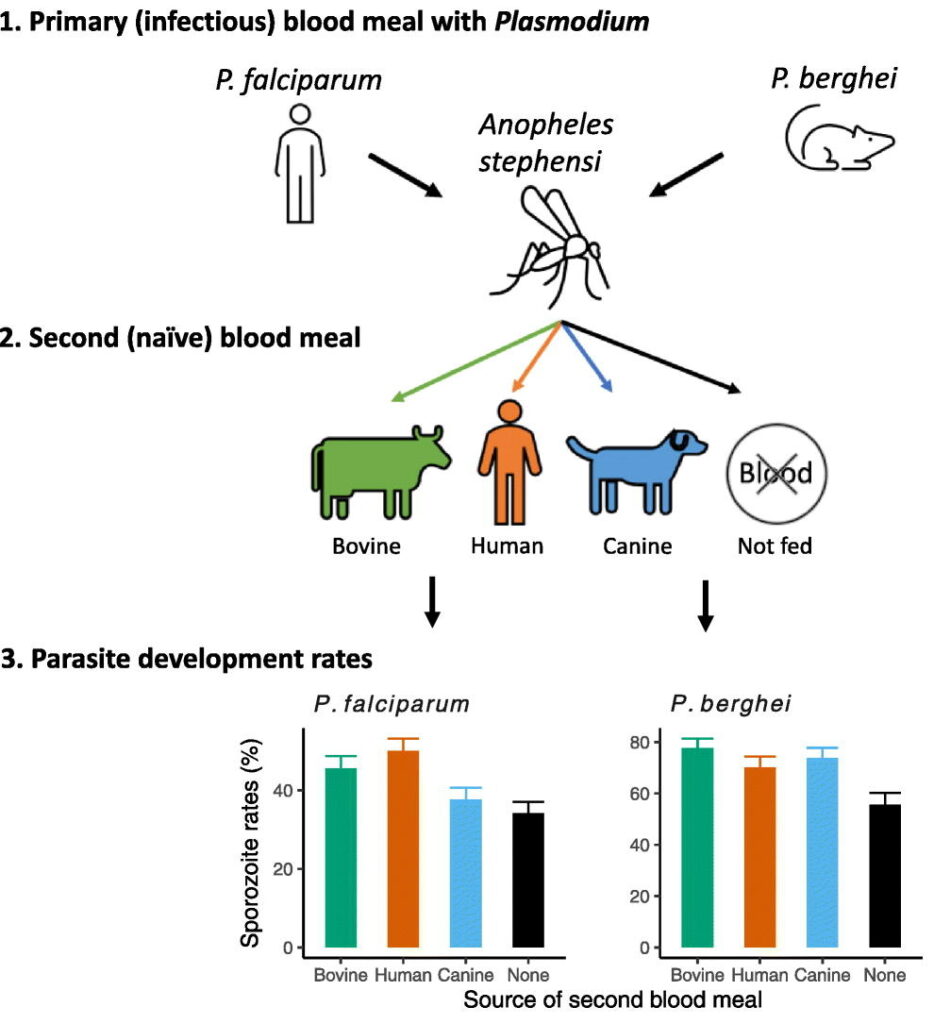
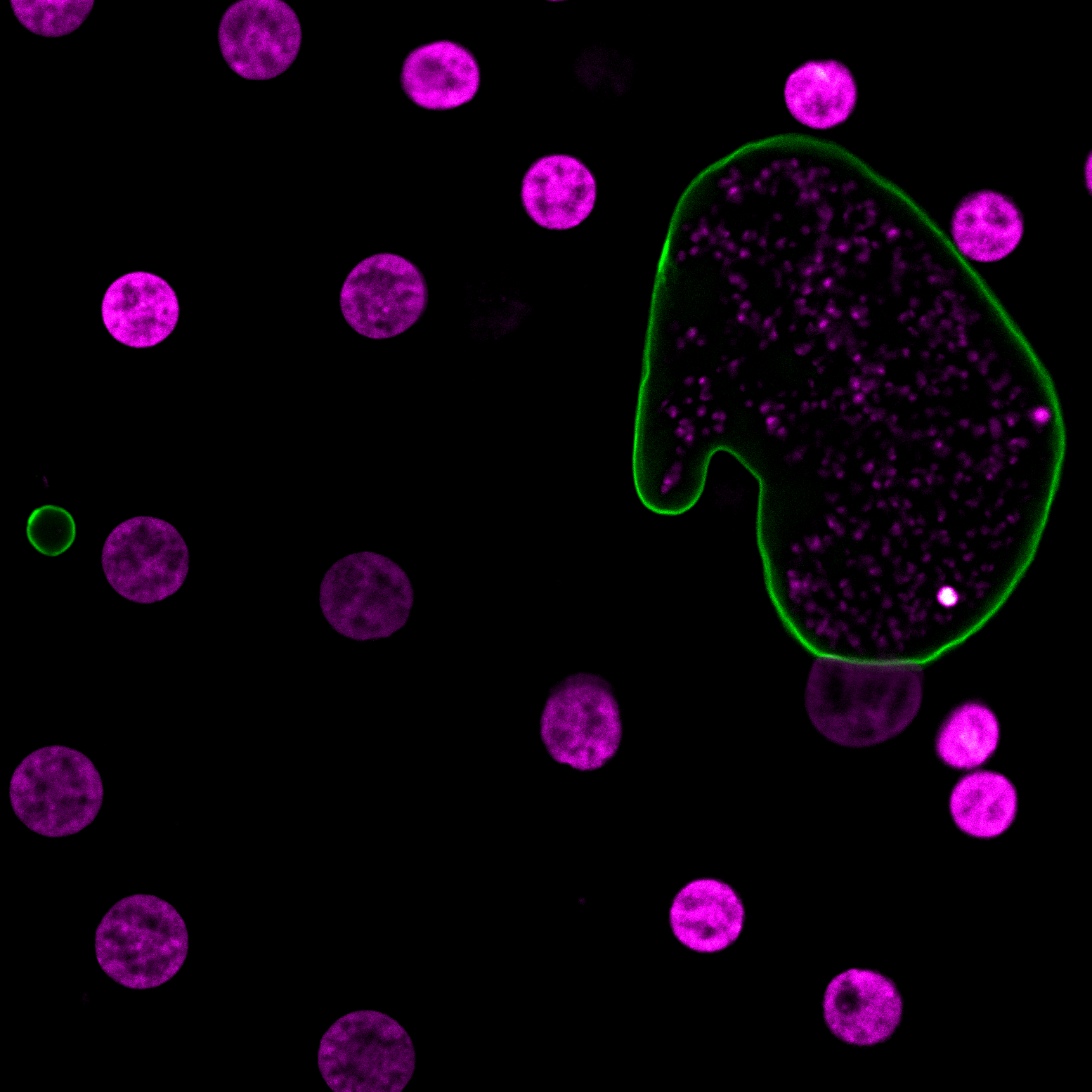
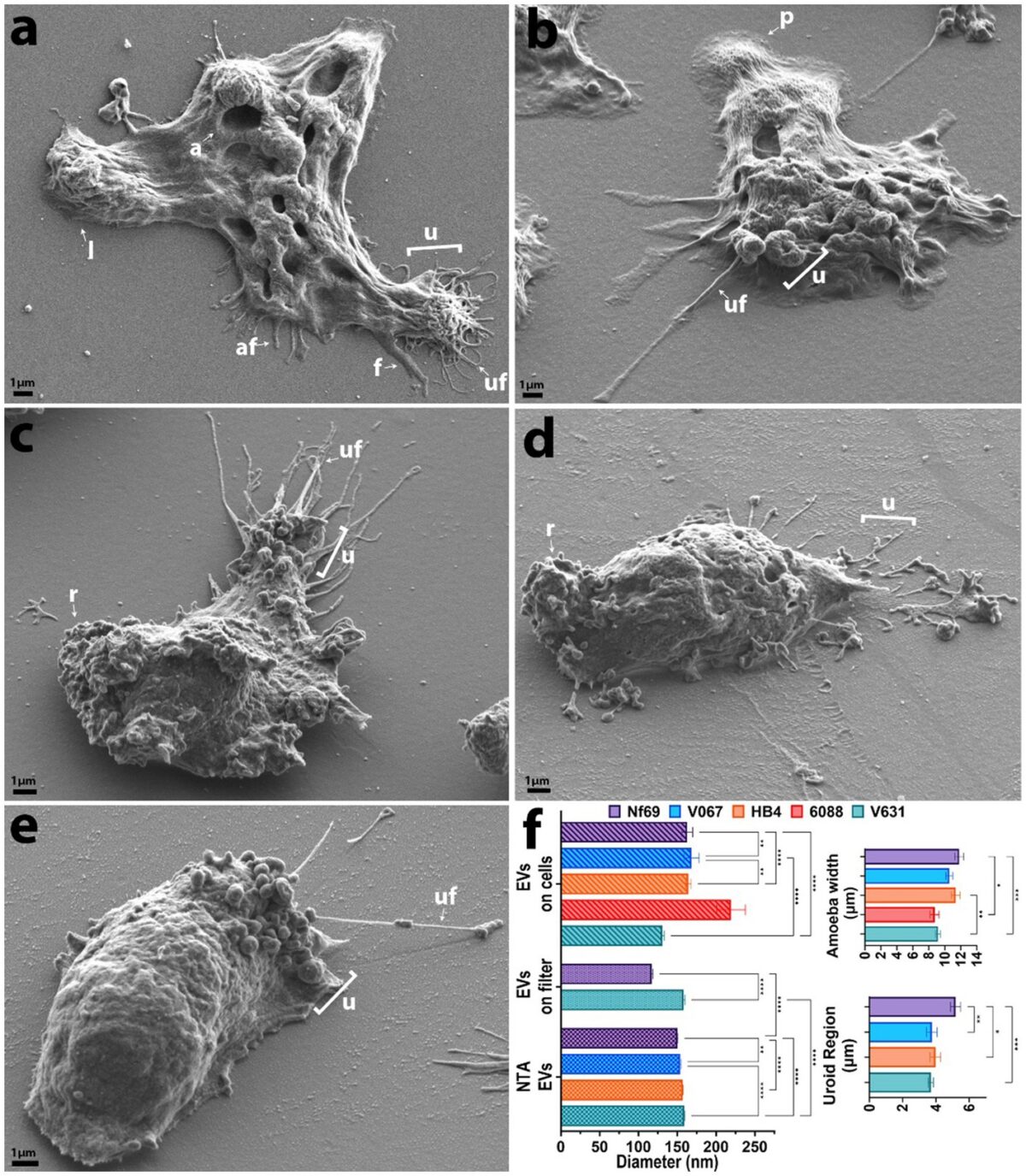
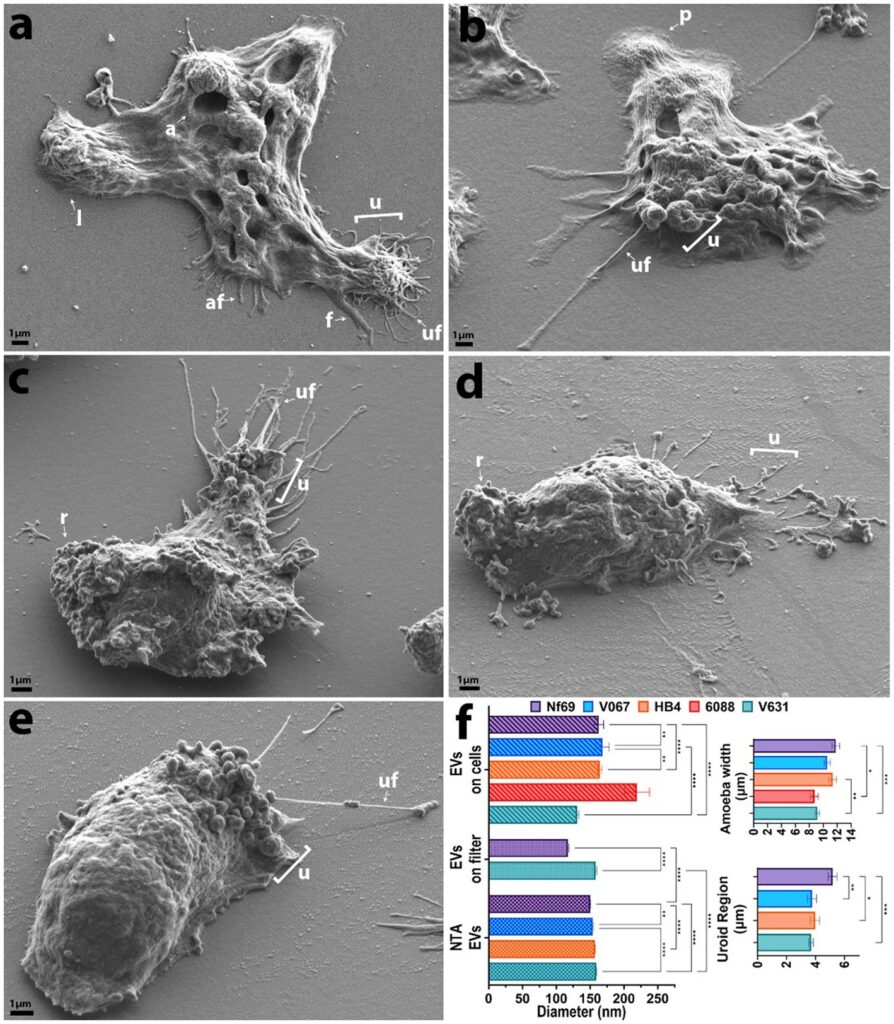
Dennis E. Kyle, professor of cellular biology and infectious diseases in the Franklin College of Arts and Sciences and the College of Veterinary Medicine, is one of the top parasitologists in the world. Kyle serves as director of the Center for Tropical and Emerging Global Diseases, and some of his most recent work focuses on discovery of new drugs that eliminate dormant vivax malaria that can linger in the liver. His group has discovered new drug series that target the dormant liver stages and is moving these novel therapeutics through preclinical studies. He also works on Naegleria fowleri, a rare but deadly parasite known as “brain-eating amoebae.” More than 97% of people infected with these amoebae die within two weeks. Kyle has conducted research into that pathogen, leading to effective repurposed drugs and the first rapid, sensitive diagnostic method.
First appeared in 2024 Research Awards