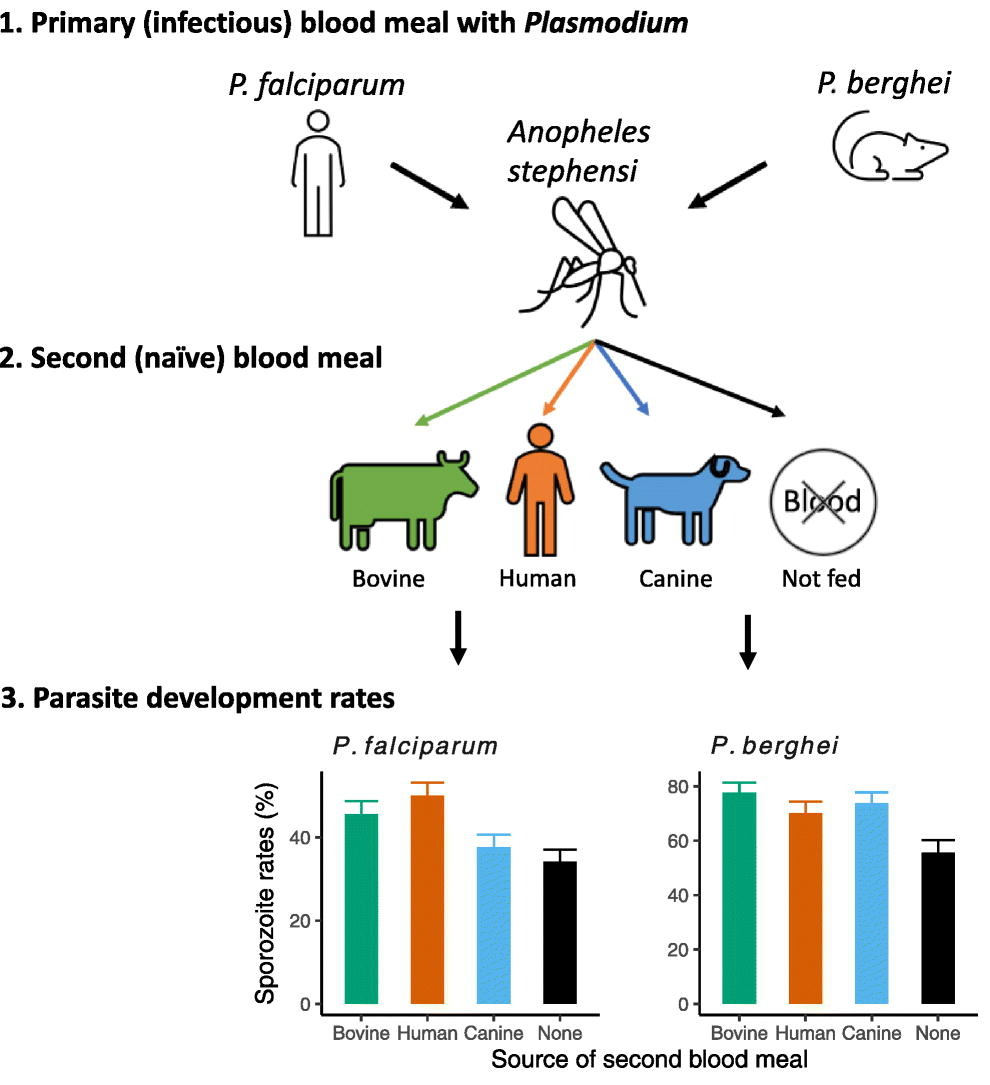
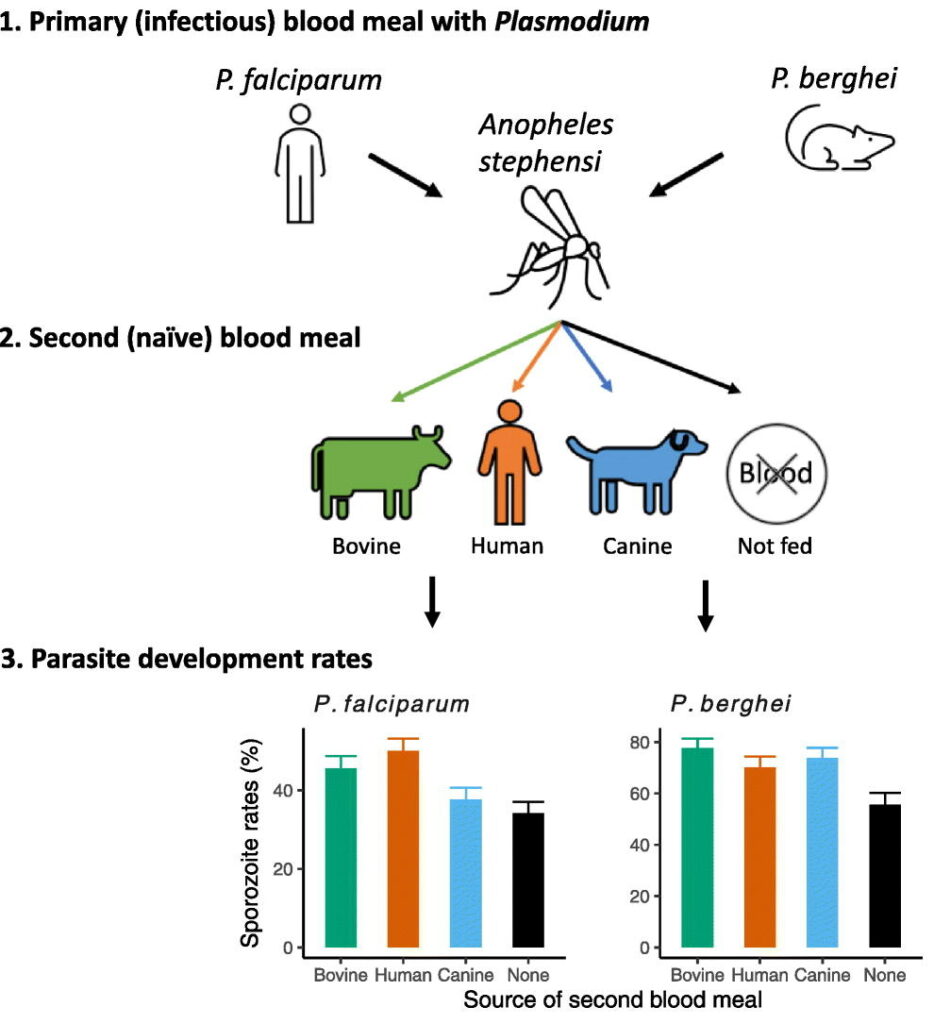
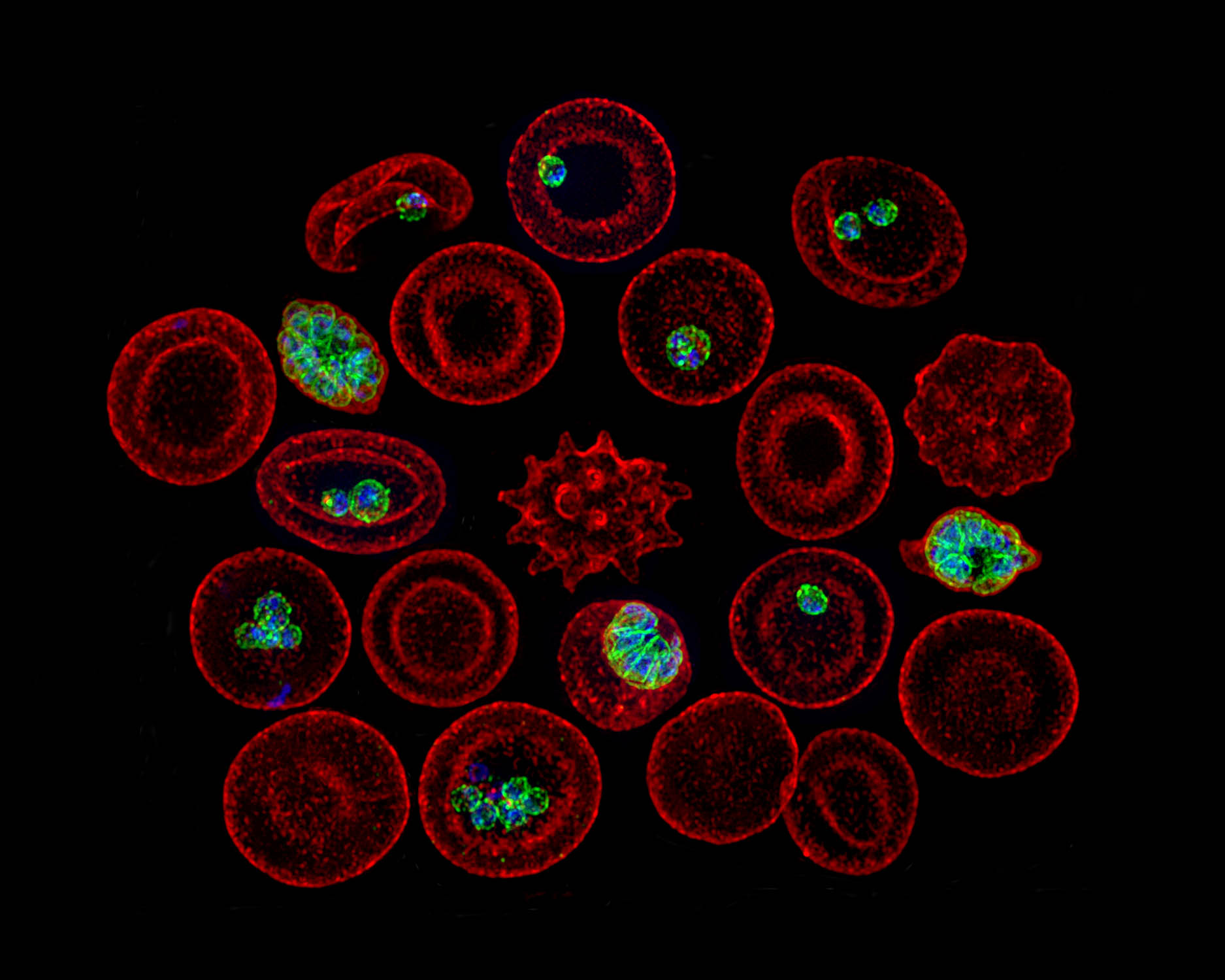
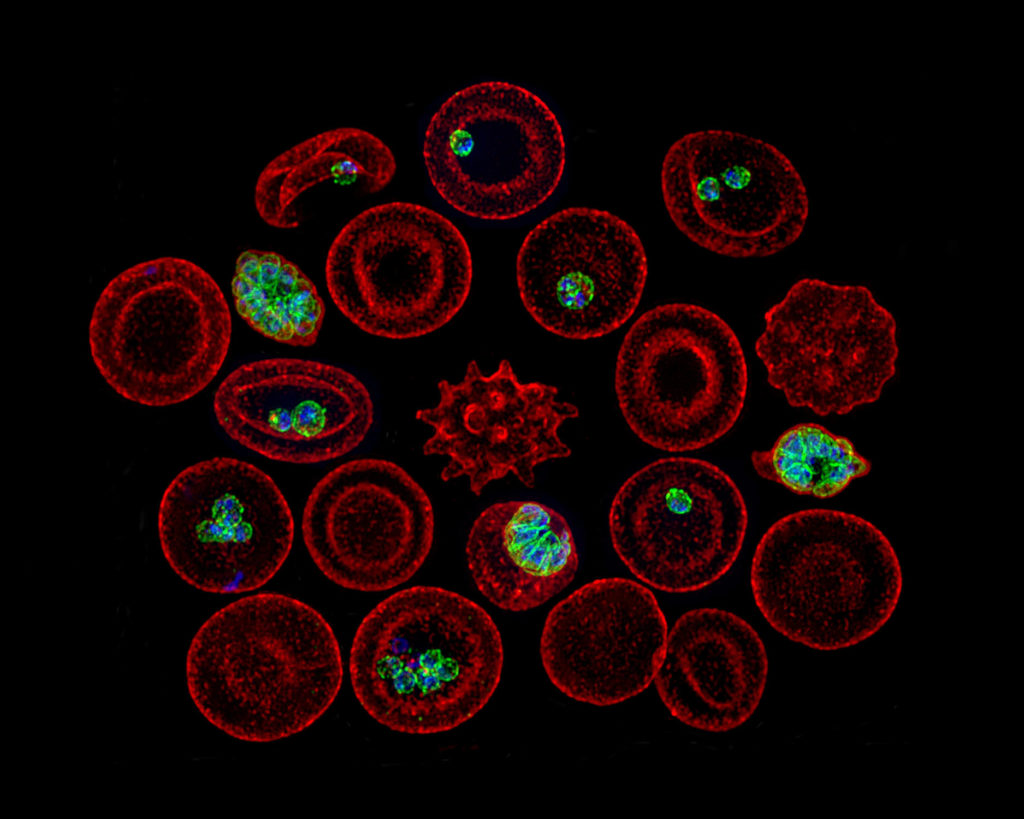
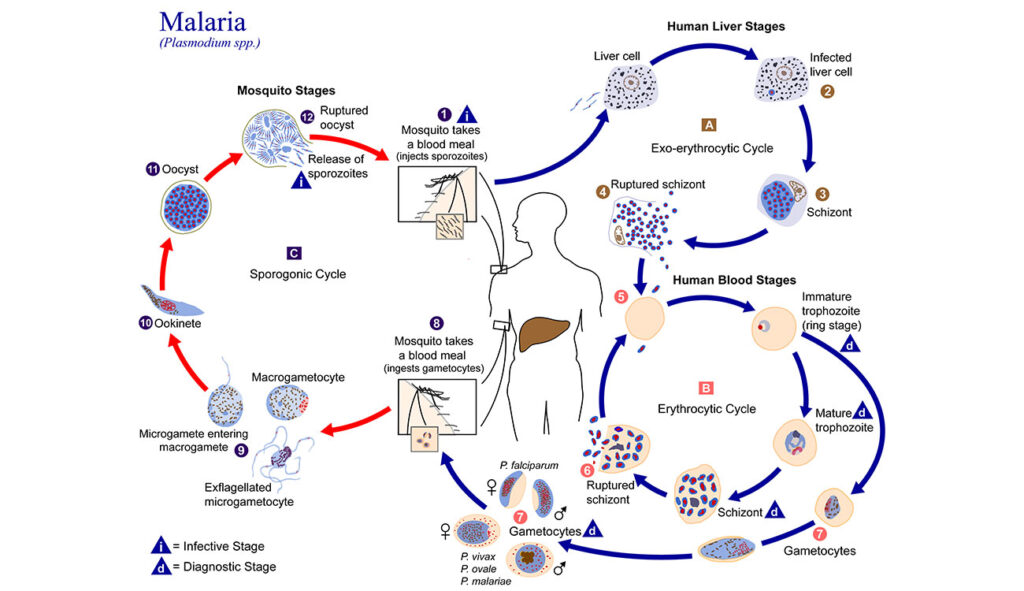
Blood meals from ‘dead-end’ vertebrate hosts enhance transmission potential of malaria-infected mosquitoes
Ingestion of an additional blood meal(s) by a hematophagic insect can accelerate development of several vector-borne parasites and pathogens. Most studies, however, offer blood from the same vertebrate host species as the original challenge (for e.g., human for primary and additional blood meals). Here, we show a second blood meal from bovine and canine hosts can also enhance sporozoite migration in Anopheles stephensi mosquitoes infected with the human- and rodent-restricted Plasmodium falciparum and P. berghei, respectively. The extrinsic incubation period (time to sporozoite appearance in salivary glands) showed more consistent reductions with blood from human and bovine donors than canine blood, although the latter’s effect may be confounded by the toxicity, albeit non-specific, associated with the anticoagulant used to collect whole blood from donors. The complex patterns of enhancement highlight the limitations of a laboratory system but are nonetheless reminiscent of parasite host-specificity and mosquito adaptations, and the genetic predisposition of An. stephensi for bovine blood. We suggest that in natural settings, a blood meal from any vertebrate host could accentuate the risk of human infections by P. falciparum: targeting vectors that also feed on animals, via endectocides for instance, may reduce the number of malaria-infected mosquitoes and thus directly lower residual transmission. Since endectocides also benefit animal health, our results underscore the utility of the One Health framework, which postulates that human health and well-being is interconnected with that of animals. We posit this framework will be further validated if our observations also apply to other vector-borne diseases which together are responsible for some of the highest rates of morbidity and mortality in socio-economically disadvantaged populations.
Ashutosh K Pathak, Justine C Shiau, Rafael C S Freitas, Dennis E Kyle. One Health. 2023 Jun 9:17:100582. doi: 10.1016/j.onehlt.2023.100582. eCollection 2023 Dec.