Plasmodium knowlesi Cytoadhesion Involves SICA Variant Proteins
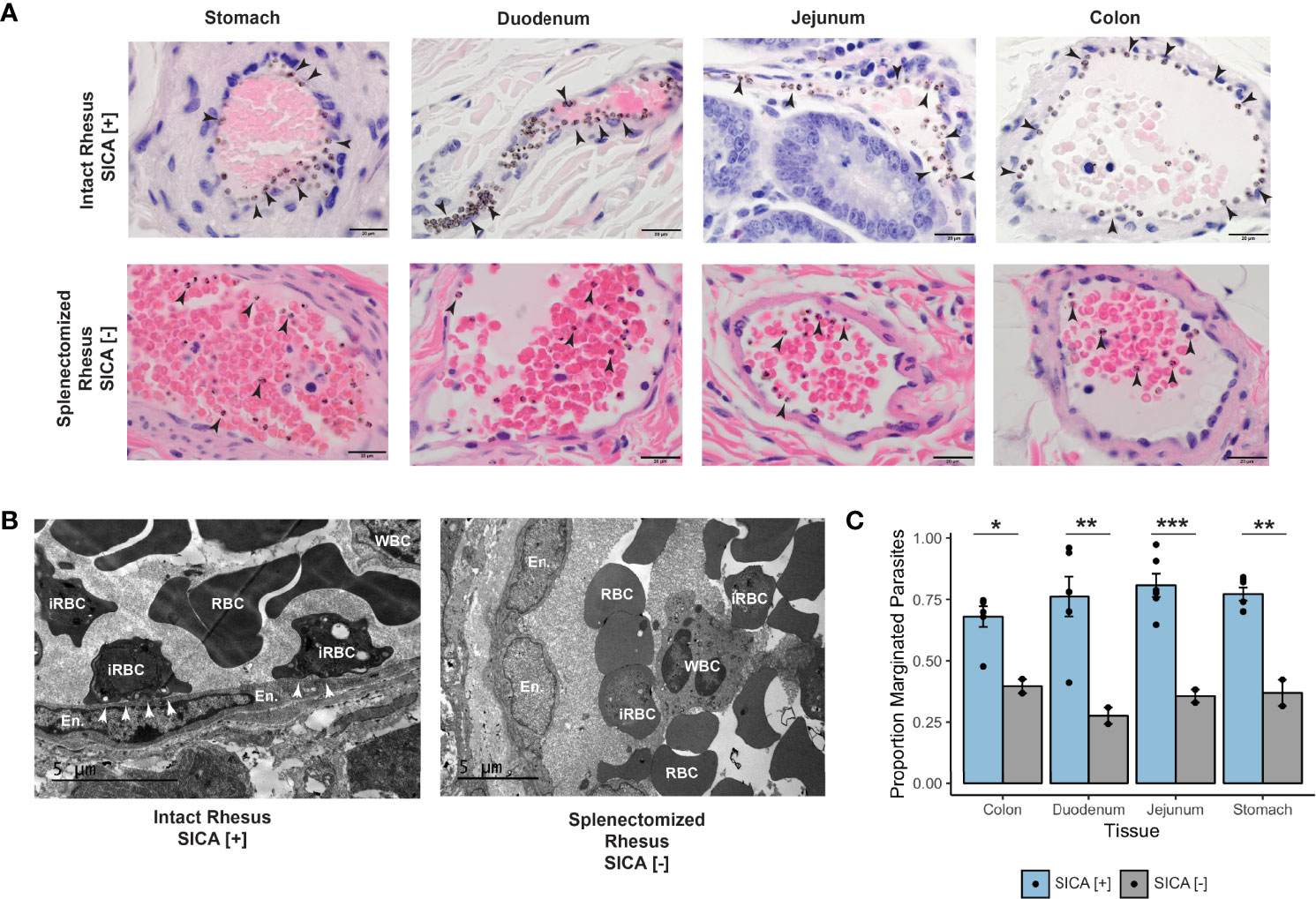
Plasmodium knowlesi poses a health threat throughout Southeast Asian communities and currently causes most cases of malaria in Malaysia. This zoonotic parasite species has been studied in Macaca mulatta (rhesus monkeys) as a model for severe malarial infections, chronicity, and antigenic variation. The phenomenon of Plasmodium antigenic variation was first recognized during rhesus monkey infections. Plasmodium-encoded variant proteins were first discovered in this species and found to be expressed at the surface of infected erythrocytes, and then named the Schizont-Infected Cell Agglutination (SICA) antigens. SICA expression was shown to be spleen dependent, as SICA expression is lost after P. knowlesi is passaged in splenectomized rhesus. Here we present data from longitudinal P. knowlesi infections in rhesus with the most comprehensive analysis to date of clinical parameters and infected red blood cell sequestration in the vasculature of tissues from 22 organs. Based on the histopathological analysis of 22 tissue types from 11 rhesus monkeys, we show a comparative distribution of parasitized erythrocytes and the degree of margination of the infected erythrocytes with the endothelium. Interestingly, there was a significantly higher burden of parasites in the gastrointestinal tissues, and extensive margination of the parasites along the endothelium, which may help explain gastrointestinal symptoms frequently reported by patients with P. knowlesi malarial infections. Moreover, this margination was not observed in splenectomized rhesus that were infected with parasites not expressing the SICA proteins. This work provides data that directly supports the view that a subpopulation of P. knowlesi parasites cytoadheres and sequesters, likely via SICA variant antigens acting as ligands. This process is akin to the cytoadhesive function of the related variant antigen proteins, namely Erythrocyte Membrane Protein-1, expressed by Plasmodium falciparum.
Mariko S Peterson, Chester J Joyner, Stacey A Lapp, Jessica A Brady, Jennifer S Wood, Monica Cabrera-Mora, Celia L Saney, Luis L Fonseca, Wayne T Cheng, Jianlin Jiang, Stephanie R Soderberg, Mustafa V Nural, Allison Hankus, Deepa Machiah, Ebru Karpuzoglu, Jeremy D DeBarry, Rabindra Tirouvanziam, Jessica C Kissinger, Alberto Moreno, Sanjeev Gumber, Eberhard O Voit, Juan B Gutierrez, Regina Joice Cordy, Mary R Galinski. Front Cell Infect Microbiol. 2022 Jun 23;12:888496. doi: 10.3389/fcimb.2022.888496. eCollection 2022.
