Optimization of diastereomeric dihydropyridines as antimalarials
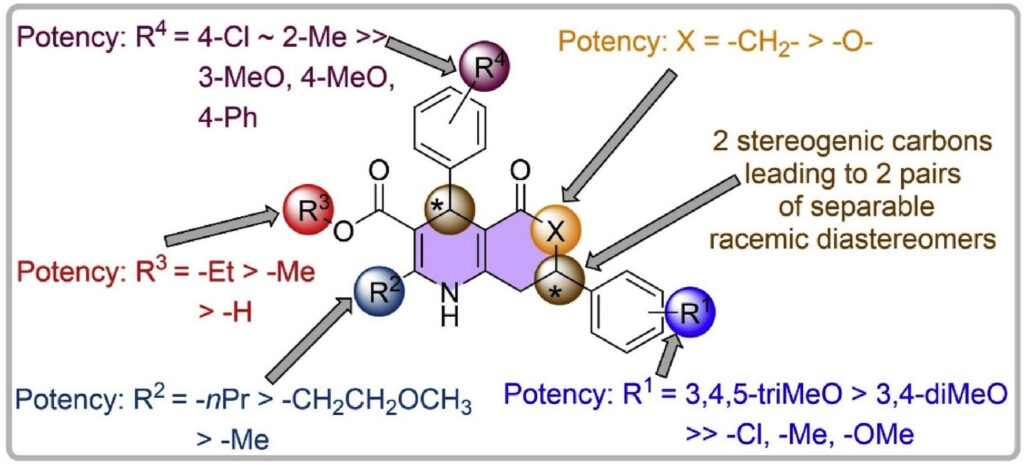
The increase in research funding for the development of antimalarials since 2000 has led to a surge of new chemotypes with potent antimalarial activity. High-throughput screens have delivered several thousand new active compounds in several hundred series, including the 4,7-diphenyl-1,4,5,6,7,8-hexahydroquinolines, hereafter termed dihydropyridines (DHPs). We optimized the DHPs for antimalarial activity. Structure-activity relationship studies focusing on the 2-, 3-, 4-, 6-, and 7-positions of the DHP core led to the identification of compounds potent (EC50 < 10 nM) against all strains of P. falciparum tested, including the drug-resistant parasite strains K1, W2, and TM90-C2B. Evaluation of efficacy of several compounds in vivo identified two compounds that reduced parasitemia by >75 % in mice 6 days post-exposure following a single 50 mg/kg oral dose. Resistance acquisition experiments with a selected dihydropyridine led to the identification of a single mutation conveying resistance in the gene encoding for Plasmodium falciparum multi-drug resistance protein 1 (PfMDR1). The same dihydropyridine possessed transmission blocking activity. The DHPs have the potential for the development of novel antimalarial drug candidates.
Kurt S Van Horn, Yingzhao Zhao, Prakash T Parvatkar, Julie Maier, Tina Mutka, Alexis Lacrue, Fabian Brockmeier, Daniel Ebert, Wesley Wu, Debora R Casandra, Niranjan Namelikonda, Jeanine Yacoub, Martina Sigal, Spencer Knapp, David Floyd, David Waterson, Jeremy N Burrows, James Duffy, Joseph L DeRisi, Dennis E Kyle, R Kiplin Guy, Roman Manetsch. Eur J Med Chem. 2024 Jun 18:275:116599. doi: 10.1016/j.ejmech.2024.116599.