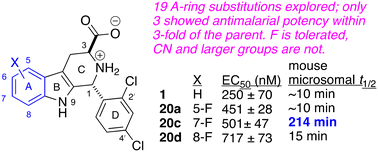
Activity of Antibacterial/Antifungal Compounds against the Protozoan Parasite, Toxoplasma gondii
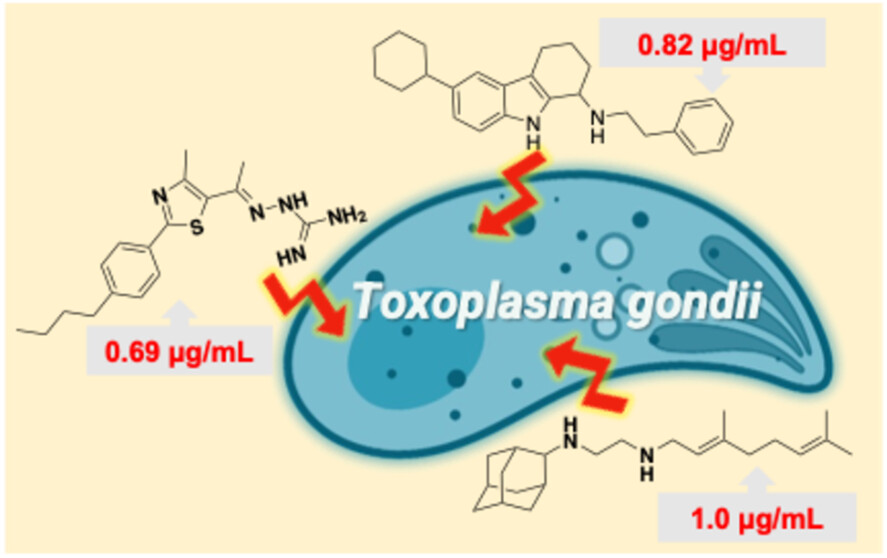
We investigated the antiparasitic activity of several antimicrobial drug leads against Toxoplasma gondii tachyzoites and, in one case, bradyzoites. Carbazole and phenylthiazole aminoguanidine anti-infectives, originally developed as antibacterial and antifungal agents, showed potent activity, with IC50 values as low as 2 μM. This potency was comparable to that observed with the tuberculosis drug candidate SQ109 and a series of its analogs. Notably, SQ109 also significantly reduced the viability of in vivo-derived bradyzoites. All compounds acted, at least in part, as protonophore uncouplers by collapsing the ΔpH component of the proton motive force. Furthermore, SQ109 and the tetrahydrocarbazole (THCz) compounds disrupted the mitochondrial membrane potential in T. gondii tachyzoites. While SQ109 is known to activate macrophages to an M1 phenotype, we observed no significant difference in its activity against T. gondii grown in fibroblasts versus macrophages, likely due to the parasite’s residence within the protective parasitophorous vacuole. We also examined correlations between compound activity against the yeast Saccharomyces cerevisiae, and the bacterium Mycobacterium smegmatis, finding significant correlations between the collapse of the proton motive force and antiproliferative activity. Taken together, our findings underscore the potential of these antimicrobial agents as promising leads for the development of new antiparasitic therapies against T. gondii.
Davinder Singh, Melissa A Sleda, Satish R Malwal, Akanksha M Pandey, Yiyuan Chen, Ruijie Zhou, Feyisola Adewole, Katie Sadowska, Oluseye K Onajole, Silvia N J Moreno, Eric Oldfield. ACS Infect Dis. 2025 Aug 28. doi: 10.1021/acsinfecdis.5c00609.