Plastid Biogenesis in Malaria Parasites Requires the Interactions and Catalytic Activity of the Clp Proteolytic System
The human malaria parasite, Plasmodium falciparum, contains an essential plastid called the apicoplast. Most apicoplast proteins are encoded by the nuclear genome and it is unclear how the plastid proteome is regulated. Here, we study an apicoplast-localized caseinolytic-protease (Clp) system and how it regulates organelle proteostasis. Using null and conditional mutants, we demonstrate that the P. falciparum Clp protease (PfClpP) has robust enzymatic activity that is essential for apicoplast biogenesis. We developed a CRISPR/Cas9-based system to express catalytically dead PfClpP, which showed that PfClpP oligomerizes as a zymogen and is matured via transautocatalysis. The expression of both wild-type and mutant Clp chaperone (PfClpC) variants revealed a functional chaperone-protease interaction. Conditional mutants of the substrate-adaptor (PfClpS) demonstrated its essential function in plastid biogenesis. A combination of multiple affinity purification screens identified the Clp complex composition as well as putative Clp substrates. This comprehensive study reveals the molecular composition and interactions influencing the proteolytic function of the apicoplast Clp system and demonstrates its central role in the biogenesis of the plastid in malaria parasites.
Anat Florentin, Dylon R. Stephens, Carrie F. Brooks, Rodrigo P. Baptista, and Vasant Muralidharan. Proc Natl Acad Sci USA. 2020 Jun 1;201919501. doi: 10.1073/pnas.1919501117.
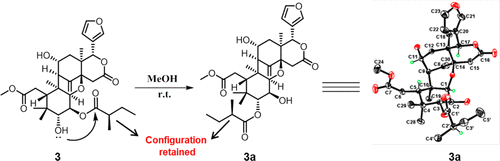
Limonoids From Cipadessa baccifera
Eighteen new limonoids, including eight methyl angolensates (1–8) and 10 cipadesins (9–18), were isolated from the leaves of Cipadessa baccifera. Their structures were characterized by means of spectroscopic data analyses, single-crystal X-ray diffraction, and quantum chemistry computational methods. The C-6 configurations in those compounds possessing a C-6 hydroxy group were all assigned as S regardless of the magnitude of J5,6, and the C-2′ configuration in those bearing a 2-methylbutyryl residue was defined by single-crystal X-ray diffraction and NMR data. Compounds 1, 5, 6, 7, 11, and 12 showed moderate antimalarial activities with IC50 values ranging from 12 to 28 μM.
Jin-Hai Yu, Hua Zhang, Bin Zhou, Flavia M. Zimbres, Seema Dalal, Qun-Fang Liu, Maria B. Cassera, and Jian-Min Yue. J Nat Prod. 2020 May 29. doi: 10.1021/acs.jnatprod.9b00666.
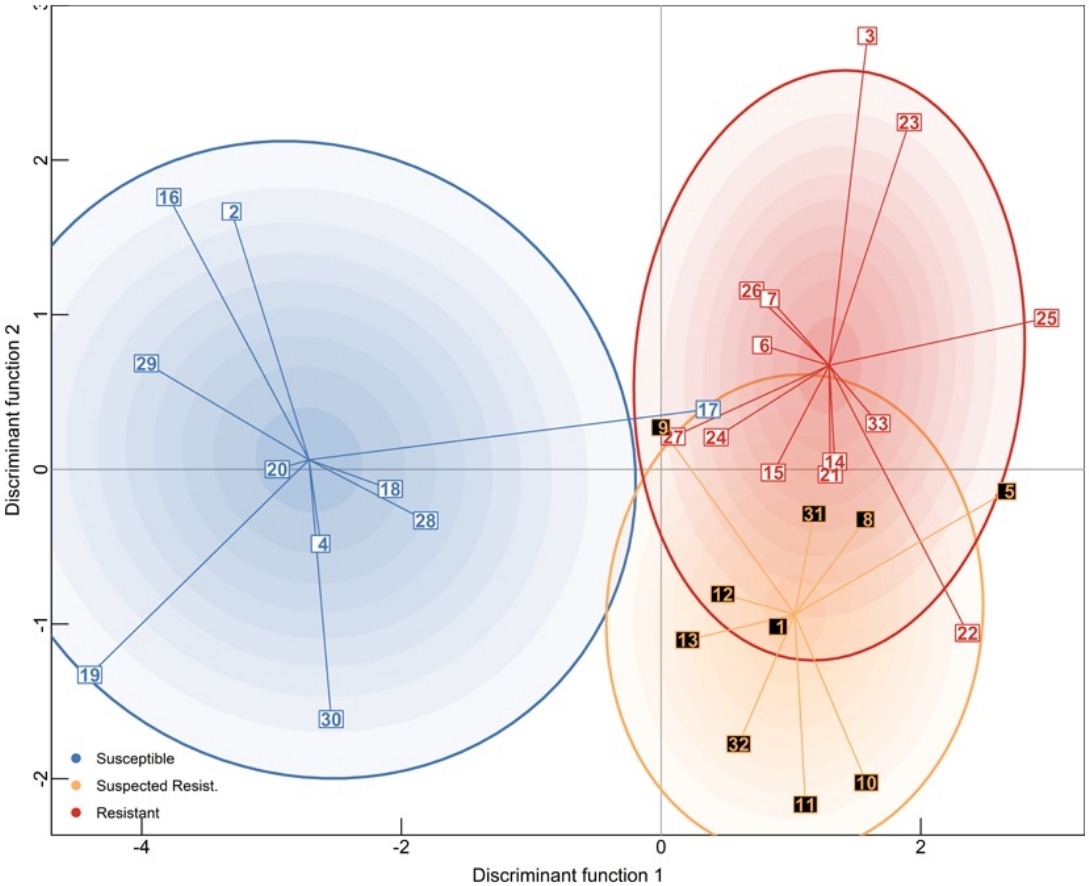
Using Population Genetics to Examine Relationships of Dirofilaria Immitis Based on Both Macrocyclic Lactone-Resistance Status and Geography
Prevention of infection with canine heartworm (Dirofilaria immitis) is based on the compliant administration of macrocyclic lactone (ML) drugs. Resistance to ML drugs is well documented in D. immitis; however, there remains a paucity of information on the spatial distribution and prevalence of resistant isolates. This project aims to improve understanding of ML-resistance by using a population genetic approach. We developed a large panel of microsatellite loci and identified 12 novel highly polymorphic markers. These 12, and five previously published markers were used to screen pools of microfilariae from 16 confirmed drug-susceptible, 25 confirmed drug-resistant, and from 10 suspected drug-resistant field isolates. In isolates where microfilarial suppression testing indicated resistance, Spatial Principal Component Analysis (sPCoA), Neighbor Joining Trees and Bayesian clustering all revealed high genetic similarity between pre- and post-treatment samples. Somewhat surprisingly, the Neighbor Joining tree and sPCoA generated using pairwise Nei’s distances did not reveal clustering for resistant isolates, nor did it reveal state-level geographic clustering from samples collected in Georgia, Louisiana or Mississippi. In contrast, Discriminant Analysis of Principle Components was able to discriminate between susceptible, suspected-resistant and resistant samples. However, no resistance-associated markers were detected, and this clustering was driven by the combined effects of multiple alleles across multiple loci. Additionally, we measured unexpectedly large genetic distances between different passages of laboratory strains that originated from the same source infection. This finding strongly suggests that the genetic makeup of laboratory isolates can change substantially with each passage, likely due to genetic bottlenecking. Taken together, these data suggest greater than expected genetic variability in the resistant isolates, and in D. immitis overall. Our results also suggest that microsatellite genotyping lacks the sensitivity to detect a specific genetic signature for resistance. Future investigations using genomic analyses will be required to elucidate the genetic relationships of ML-resistant isolates.
Julie Sanchez, Guha Dharmarajan, Melissa M. George, Cassan Pulaski, Adrian J. Wolstenholme, John S. Gilleard, Ray M. Kaplan. 2020 Veterinary Parasitology; 283:109125. doi: 10.1016/j.vetpar.2020.109125.
Combination of Serological, Antigen Detection, and DNA Data for Plasmodium Falciparum Provides Robust Geospatial Estimates for Malaria Transmission in Haiti
Microscopy is the gold standard for malaria epidemiology, but laboratory and point-of-care (POC) tests detecting parasite antigen, DNA, and human antibodies against malaria have expanded this capacity. The island nation of Haiti is endemic for Plasmodium falciparum (Pf) malaria, though at a low national prevalence and heterogenous geospatial distribution. In 2015 and 2016, serosurveys were performed of children (ages 6-7 years) sampled in schools in Saut d’Eau commune (n = 1,230) and Grand Anse department (n = 1,664) of Haiti. Children received malaria antigen rapid diagnostic test and provided a filter paper blood sample for further laboratory analysis of the Pf histidine-rich protein 2 (HRP2) antigen, Pf DNA, and anti-Pf IgG antibodies. Prevalence of Pf infection ranged from 0.0-16.7% in 53 Saut d’Eau schools, and 0.0-23.8% in 56 Grand Anse schools. Anti-Pf antibody carriage exceeded 80% of students in some schools from both study sites. Geospatial prediction ellipses were created to indicate clustering of positive tests within the survey areas and overlay of all prediction ellipses for the different types of data revealed regions with high likelihood of active and ongoing Pf malaria transmission. The geospatial utilization of different types of Pf data can provide high confidence for spatial epidemiology of the parasite.
Adan Oviedo, Alaine Knipes, Caitlin Worrell, LeAnne M Fox, Luccene Desir, Carl Fayette, Alain Javel, Franck Monestime, Kimberly Mace, Michelle A Chang, Venkatachalam Udhayakumar, Jean F Lemoine, Kimberly Won, Patrick J Lammie, Eric Rogier. Scientific Reports volume 10, Article number: 8443 (2020). https://doi.org/10.1038/s41598-020-65419-w
May 2020 Newsletter

The May CTEGD Newsletter is now available.
In this issue…
Faculty Honors
Trainee Updates
Research news
Grants
Giving Opportunities
CTEGD names travel fund for Daniel G. Colley

One day Daniel Colley raised his hand to volunteer, setting in motion five decades of scientific adventures. It was 1969, and Colley’s postdoctoral adviser, ByronWaksman, a renowned immunologist at Yale University School of Medicine, had stepped into the laboratory and asked if anyone wanted to go to Brazil. Colley, today a UGA immunologist and Fellow of the American Association for the Advancement of Science, became fascinated by schistosomiasis, a parasitic worm infection plaguing poverty-stricken communities in sub-Saharan Africa and around the world.
After his Brazil sojourn, Colley arrived at Vanderbilt University in 1971 to begin setting up a lab and a career-long effort to understand the immunological paradox of schistosomiasis. In 1992, he joined the Centers for Disease Control and Prevention (CDC) and a year later was promoted to director of the Division of Parasitic Diseases. After retiring from the CDC, he arrived at UGA in 2001 as professor of microbiology and director of the Center for Tropical and Emerging Global Diseases. During the past decade, Colley has been director of UGA’s Schistosomiasis Consortium for Operational Research and Evaluation (SCORE). In June 2020, Colley retired from the University of Georgia. He has been named Professor Emeritus.
That trip to Brazil was instrumental in shaping Colley’s career. As a mentor, he is passionate about providing the same opportunity to new scientists. Early in his career at UGA, he established the Training Innovations in Parasitological Studies (TIPS) fellowship through funding from the Ellison Medical Foundation, funding that has since ended. In honor of Colley’s commitment to understanding diseases of poverty and training the next generation of scientists, the Center for Tropical and Emerging Global Diseases is establishing the Daniel G. Colley Training in Parasitology Fund to continue his legacy.
[button size=’large’ style=” text=’Give Now’ icon=” icon_color=’BA0C2F’ link=’https://gail.uga.edu/giving/as/daniel-colley-fund’ target=’_self’ color=” hover_color=” border_color=” hover_border_color=” background_color=” hover_background_color=” font_style=” font_weight=” text_align=’center’ margin=”]
Related news: Professor Emeritus Dan Colley to discuss 50 years of Schistosoma research
Nationwide Remapping of Schistosoma mansoni Infection in Rwanda Using Circulating Cathodic Antigen Rapid Test: Taking Steps Toward Elimination
Eugene Ruberanziza, Udo Wittmann, Aimable Mbituyumuremyi, Alphonse Mutabazi, Carl H. Campbell Jr, Daniel G. Colley, Fiona M. Fleming, Giuseppina Ortu, Govert J. van Dam, Irenee Umulisa, Jamie Tallant, Michee Kabera, Muhammed Semakula, Paul L. A. M. Corstjens, Tharcisse Munyaneza, Warren Lancaster, Jean Bosco Mbonigaba and Michelle N. Clements. Am J Trop Med Hyg. 2020 May 18. doi: 10.4269/ajtmh.19-0866.