ATP synthase-associated coiled-coil-helix-coiled-coil-helix (CHCH) domain-containing proteins are critical for mitochondrial function in Toxoplasma gondii
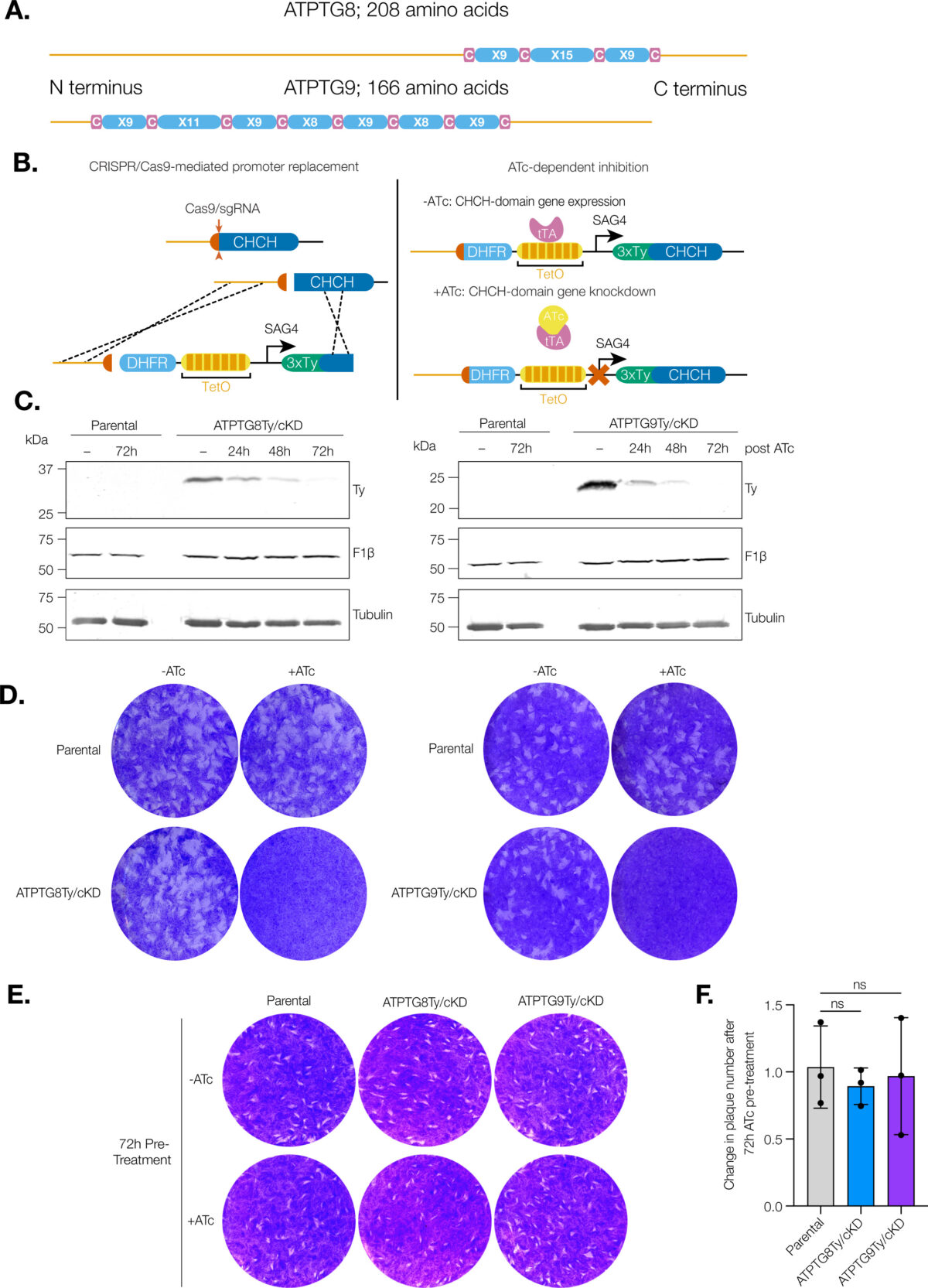
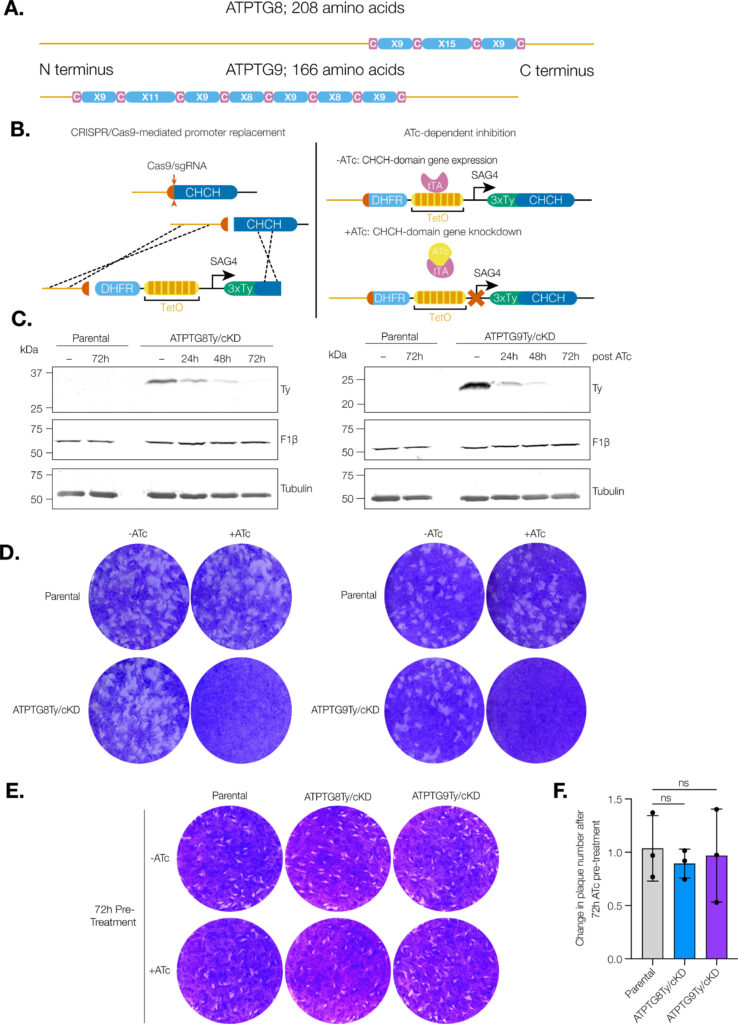
Coiled-coil-helix-coiled-coil-helix (CHCH) domains consist of two pairs of cysteine residues that are oxidized to form disulfide bonds upon mitochondrial import. Proteins containing these domains play important roles in mitochondrial ultrastructure and in the biogenesis, function, and stability of electron transport chain complexes. Interestingly, recent investigations of the Toxoplasma gondii ATP synthase identified subunits containing CHCH domains. As CHCH domain proteins have never been found in any other ATP synthase, their role in T. gondii was unclear. Using conditional gene knockdown systems, we investigated two T. gondii ATP synthase subunits containing CHCH domains: ATPTG8 and ATPTG9. We show that these two subunits are essential for the lytic cycle as well as stability and function of the ATP synthase. Further, we illustrated that their knockdown disrupts multiple aspects of mitochondrial morphology, including ultrastructure and cristae density. Mutation of key cysteine residues in the CHCH domains also caused mis-localization of the proteins. Our work suggests that these proteins likely provide structural support to the exceptionally large T. gondii ATP synthase complex and that perturbations to the structural integrity of this complex result in deleterious downstream effects on the parasite mitochondrion. These investigations add to a growing body of work focused on the divergent aspects of the apicomplexan ATP synthase, which could ultimately uncover novel drug targets. IMPORTANCE Members of the coiled-coil-helix-coiled-coil-helix (CHCH) domain protein family are transported into the mitochondrial intermembrane space, where they play important roles in the biogenesis and function of the organelle. Unexpectedly, the ATP synthase of the apicomplexan Toxoplasma gondii harbors CHCH domain-containing subunits of unknown function. As no other ATP synthase studied to date contains this class of proteins, characterizing their function will be of broad interest to the fields of molecular parasitology and mitochondrial evolution. Here, we demonstrate that that two T. gondii ATP synthase subunits containing CHCH domains are required for parasite survival and for stability and function of the ATP synthase. We also show that knockdown disrupts multiple aspects of the mitochondrial morphology of T. gondii and that mutation of key residues in the CHCH domains caused mis-localization of the proteins. This work provides insight into the unique features of the apicomplexan ATP synthase, which could help to develop therapeutic interventions against this parasite and other apicomplexans, such as the malaria-causing parasite Plasmodium falciparum.
Madelaine M Usey, Diego Huet. mBio. 2023 Oct 5:e0176923. doi: 10.1128/mbio.01769-23.
