Targeted Inhibition of Plasmodium falciparum Calcium-Dependent Protein Kinase 1 with a Constrained J Domain-Derived Disruptor Peptide
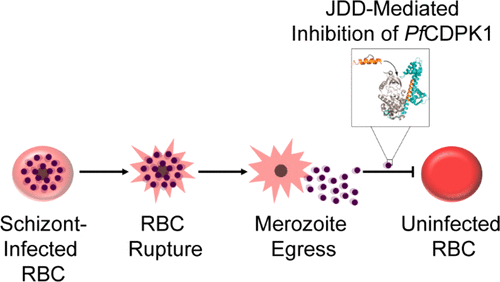
To explore the possibility of constrained peptides to target Plasmodium-infected cells, we designed a J domain mimetic derived from Plasmodium falciparum calcium-dependent protein kinase 1 ( PfCDPK1) as a strategy to disrupt J domain binding and inhibit PfCDPK1 activity. The J domain disruptor (JDD) peptide was conformationally constrained using a hydrocarbon staple and was found to selectively permeate segmented schizonts and colocalize with intracellular merozoites in late-stage parasites. In vitro analyses demonstrated that JDD could effectively inhibit the catalytic activity of recombinant PfCDPK1 in the low micromolar range. Treatment of late-stage parasites with JDD resulted in a significant decrease in parasite viability mediated by a blockage of merozoite invasion, consistent with a primary effect of PfCDPK1 inhibition. To the best of our knowledge, this marks the first use of stapled peptides designed to specifically target a Plasmodium falciparum protein and demonstrates that stapled peptides may serve as useful tools for exploring potential antimalarial agents.
Briana R. Flaherty, Tienhuei G. Ho, Sven H. Schmidt, Friedrich W. Herberg, David S. Peterson, and Eileen J. Kennedy. 2019. ACS Infectious Diseases. DOI: 10.1021/acsinfecdis.8b0034
