Liver-stage fate determination in Plasmodium vivax parasites: Characterization of schizont growth and hypnozoite fating from patient isolates
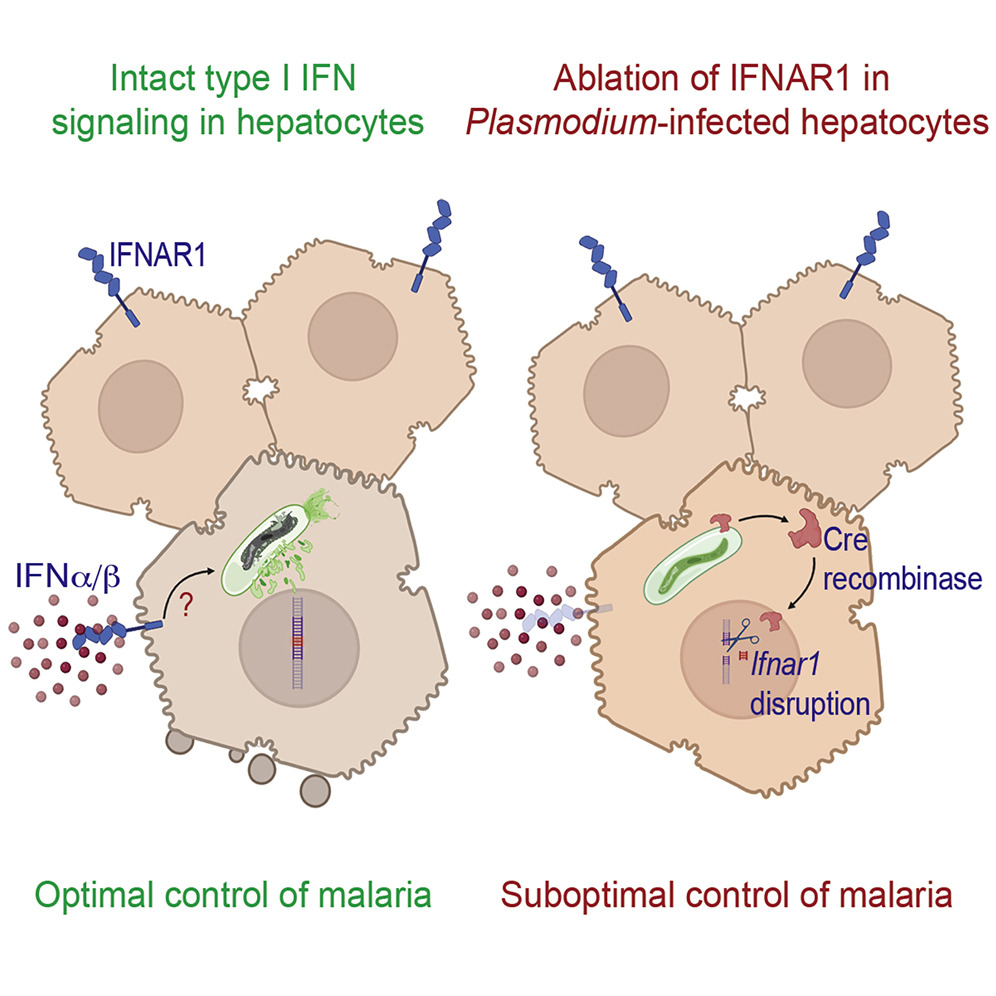
Plasmodium vivax, one species of parasite causing human malaria, forms a dormant liver stage, termed the hypnozoite, which activate weeks, months or years after the primary infection, causing relapse episodes. Relapses significantly contribute to the vivax malaria burden and are only killed with drugs of the 8-aminoquinoline class, which are contraindicated in many vulnerable populations. Development of new therapies targeting hypnozoites is hindered, in part, by the lack of robust methods to continuously culture and characterize this parasite. As a result, the determinants of relapse periodicity and the molecular processes that drive hypnozoite formation, persistence, and activation are largely unknown. While previous reports have described vastly different liver-stage growth metrics attributable to which hepatocyte donor lot is used to initiate culture, a comprehensive assessment of how different P. vivax patient isolates behave in the same lots at the same time is logistically challenging. Using our primary human hepatocyte-based P. vivax liver-stage culture platform, we aimed to simultaneously test the effects of how hepatocyte donor lot and P. vivax patient isolate influence the fate of sporozoites and growth of liver schizonts. We found that, while environmental factors such as hepatocyte donor lot can modulate hypnozoite formation rate, the P. vivax case is also an important determinant of the proportion of hypnozoites observed in culture. In addition, we found schizont growth to be mostly influenced by hepatocyte donor lot. These results suggest that, while host hepatocytes harbor characteristics making them more- or less-supportive of a quiescent versus growing intracellular parasite, sporozoite fating toward hypnozoites is isolate-specific. Future studies involving these host-parasite interactions, including characterization of individual P. vivax strains, should consider the impact of culture conditions on hypnozoite formation, in order to better understand this important part of the parasite’s lifecycle.
Amélie Vantaux, Julie Péneau, Caitlin A Cooper, Dennis E Kyle, Benoit Witkowski, Steven P Maher. Front Microbiol. 2022 Sep 23;13:976606. doi: 10.3389/fmicb.2022.976606.