Alkyne modified purines for assessment of activation of Plasmodium vivax hypnozoites and growth of pre-erythrocytic and erythrocytic stages in Plasmodium spp
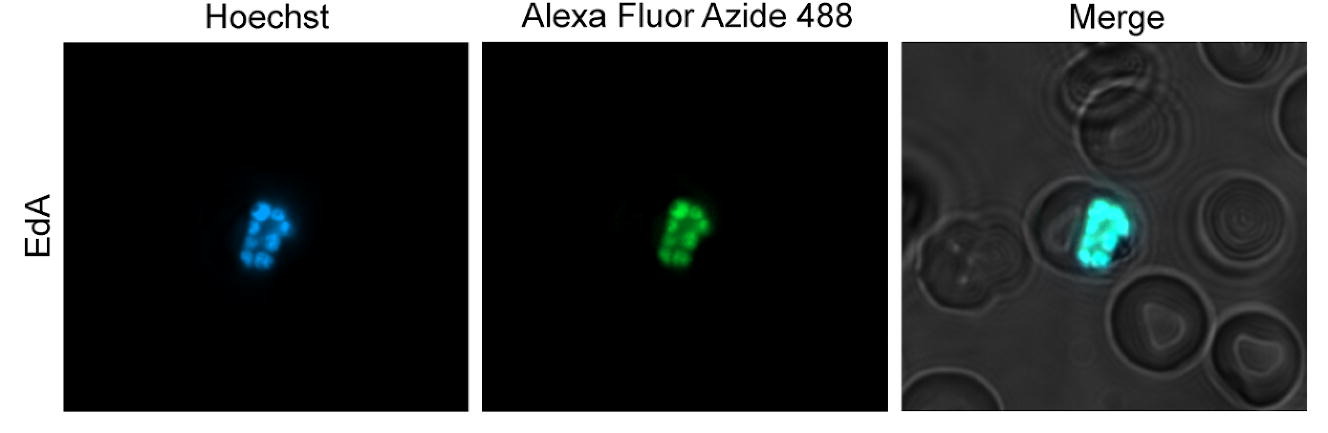
Malaria is a major global health problem which predominantly afflicts developing countries. Although many antimalarial therapies are currently available, the protozoan parasite causing this disease, Plasmodium spp., continues to evade eradication efforts. One biological phenomenon hampering eradication efforts is the parasite’s ability to arrest development, transform into a drug-insensitive form, and then resume growth post-therapy. Currently, the mechanisms by which the parasite enters arrested development, or dormancy, and later recrudesces or reactivates to continue development, are unknown and the malaria field lacks techniques to study these elusive mechanisms. Since Plasmodium spp. salvage purines for DNA synthesis, we hypothesized that alkyne-containing purine nucleosides could be used to develop a DNA synthesis marker which could be used to investigate mechanisms behind dormancy. Using copper-catalyzed click chemistry methods, we observe incorporation of alkyne modified adenosine, inosine, and hypoxanthine in actively replicating asexual blood stages of Plasmodium falciparum and incorporation of modified adenosine in actively replicating liver stage schizonts of Plasmodium vivax. Notably, these modified purines were not incorporated in dormant liver stage hypnozoites, suggesting this marker could be used as a tool to differentiate replicating and non-replicating liver forms and, more broadly, as a tool for advancing our understanding of Plasmodium dormancy mechanisms.
Alona Botnar, Grant Lawrence, Steven P Maher, Amélie Vantaux, Benoît Witkowski, Justine C Shiau, Emilio F Merino, David De Vore, Christian Yang, Cameron Murray, Maria B Cassera, James W Leahy, Dennis E Kyle. Int J Parasitol. 2022 Apr 18;S0020-7519(22)00066-2. doi: 10.1016/j.ijpara.2022.03.003.