Synergy between a cytoplasmic vWFA/VIT protein and a WD40-repeat F-box protein controls development in Dictyostelium
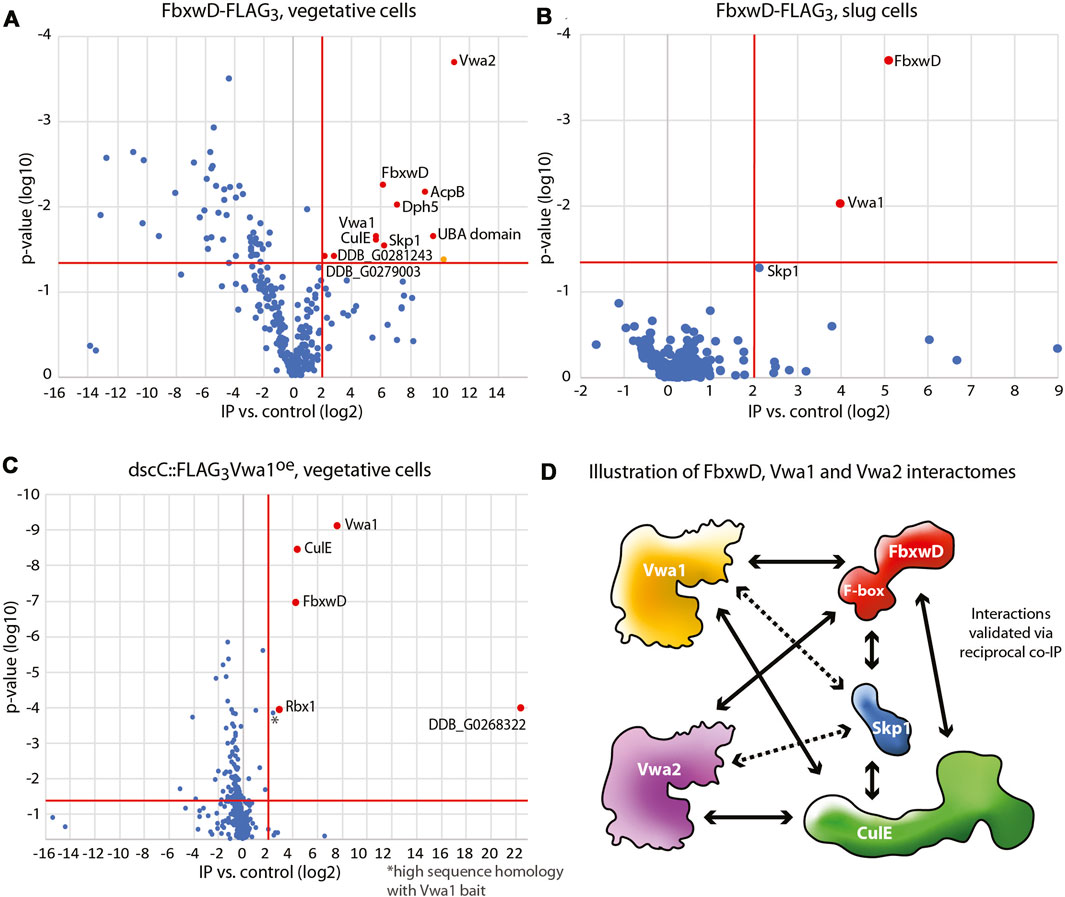
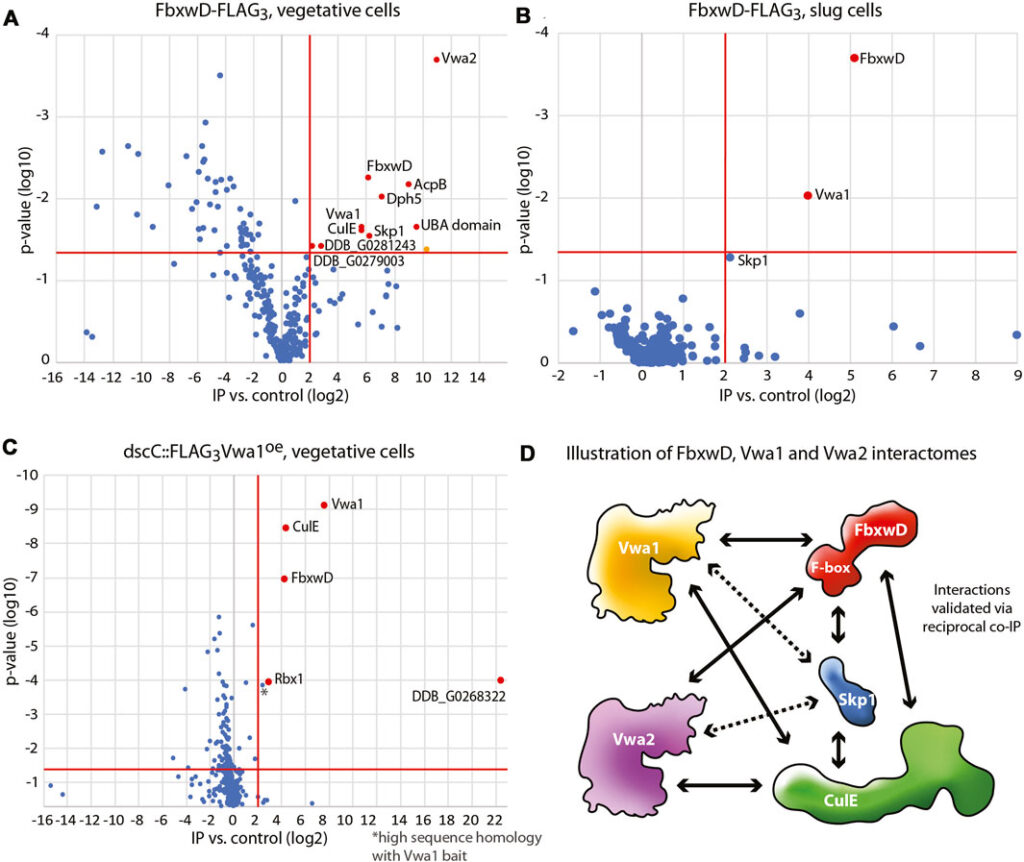
Like most eukaryotes, the pre-metazoan social amoeba Dictyostelium depends on the SCF (Skp1/cullin-1/F-box protein) family of E3 ubiquitin ligases to regulate its proteome. In Dictyostelium, starvation induces a transition from unicellular feeding to a multicellular slug that responds to external signals to culminate into a fruiting body containing terminally differentiated stalk and spore cells. These transitions are subject to regulation by F-box proteins and O2-dependent posttranslational modifications of Skp1. Here we examine in greater depth the essential role of FbxwD and Vwa1, an intracellular vault protein inter-alpha-trypsin (VIT) and von Willebrand factor-A (vWFA) domain containing protein that was found in the FbxwD interactome by co-immunoprecipitation. Reciprocal co-IPs using gene-tagged strains confirmed the interaction and similar changes in protein levels during multicellular development suggested co-functioning. FbxwD overexpression and proteasome inhibitors did not affect Vwa1 levels suggesting a non-substrate relationship. Forced FbxwD overexpression in slug tip cells where it is normally enriched interfered with terminal cell differentiation by a mechanism that depended on its F-box and RING domains, and on Vwa1 expression itself. Whereas vwa1-disruption alone did not affect development, overexpression of either of its three conserved domains arrested development but the effect depended on Vwa1 expression. Based on structure predictions, we propose that the Vwa1 domains exert their negative effect by artificially activating Vwa1 from an autoinhibited state, which in turn imbalances its synergistic function with FbxwD. Autoinhibition or homodimerization might be relevant to the poorly understood tumor suppressor role of the evolutionarily related VWA5A/BCSC-1 in humans.
Andrew W Boland, Elisabet Gas-Pascual, Hanke van der Wel, Hyun W Kim, Christopher M West. Front Cell Dev Biol. 2023 Sep 14;11:1259844. doi: 10.3389/fcell.2023.1259844. eCollection 2023.
