Multi-target heteroleptic palladium bisphosphonate complexes
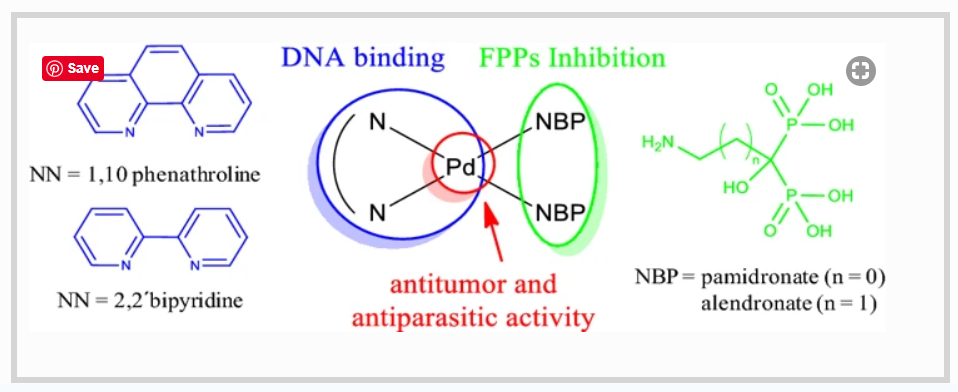
 Bisphosphonates are the most commonly prescribed drugs for the treatment of osteoporosis and other bone illnesses. Some of them have also shown antiparasitic activity. In search of improving the pharmacological profile of commercial bisphosphonates, our group had previously developed first row transition metal complexes with N-containing bisphosphonates (NBPs). In this work, we extended our studies to heteroleptic palladium–NBP complexes including DNA intercalating polypyridyl co-ligands (NN) with the aim of obtaining potential multi-target species. Complexes of the formula [Pd(NBP)2(NN)]·2NaCl·xH2O with NBP = alendronate (ale) or pamidronate (pam) and NN = 1,10 phenanthroline (phen) or 2,2′-bipyridine (bpy) were synthesized and fully characterized. All the obtained compounds were much more active in vitro against T. cruzi (amastigote form) than the corresponding NBP ligands. In addition, complexes were nontoxic to mammalian cells up to 50–100 µM. Compounds with phen as ligand were 15 times more active than their bpy analogous. Related to the potential mechanism of action, all complexes were potent inhibitors of two parasitic enzymes of the isoprenoid biosynthetic pathway. No correlation between the anti-T. cruzi activity and the enzymatic inhibition results was observed. On the contrary, the high antiparasitic activity of phen-containing complexes could be related to their ability to interact with DNA in an intercalative-like mode. These rationally designed compounds are good candidates for further studies and good leaders for future drug developments.
Bisphosphonates are the most commonly prescribed drugs for the treatment of osteoporosis and other bone illnesses. Some of them have also shown antiparasitic activity. In search of improving the pharmacological profile of commercial bisphosphonates, our group had previously developed first row transition metal complexes with N-containing bisphosphonates (NBPs). In this work, we extended our studies to heteroleptic palladium–NBP complexes including DNA intercalating polypyridyl co-ligands (NN) with the aim of obtaining potential multi-target species. Complexes of the formula [Pd(NBP)2(NN)]·2NaCl·xH2O with NBP = alendronate (ale) or pamidronate (pam) and NN = 1,10 phenanthroline (phen) or 2,2′-bipyridine (bpy) were synthesized and fully characterized. All the obtained compounds were much more active in vitro against T. cruzi (amastigote form) than the corresponding NBP ligands. In addition, complexes were nontoxic to mammalian cells up to 50–100 µM. Compounds with phen as ligand were 15 times more active than their bpy analogous. Related to the potential mechanism of action, all complexes were potent inhibitors of two parasitic enzymes of the isoprenoid biosynthetic pathway. No correlation between the anti-T. cruzi activity and the enzymatic inhibition results was observed. On the contrary, the high antiparasitic activity of phen-containing complexes could be related to their ability to interact with DNA in an intercalative-like mode. These rationally designed compounds are good candidates for further studies and good leaders for future drug developments.
Micaella Cipriani, Santiago Rostán, Ignacio León, Zhu-Hong Li, Jorge S. Gancheff, Ulrike Kemmerling, Claudio Olea Azar, Susana Etcheverry, Roberto Docampo, Dinorah Gambino & Lucía Otero. J Biol Inorg Chem. 2020 Mar 30. doi: 10.1007/s00775-020-01779-y.
