Monoclonal Antibodies Generated against Glycoconjugates Recognize Chemical Linkers
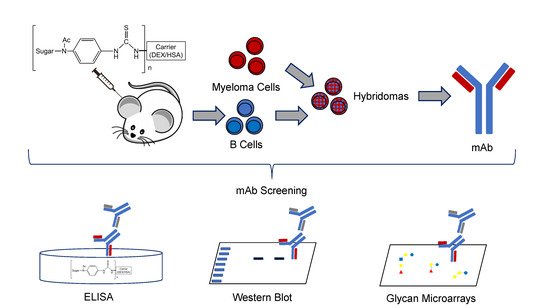
 Monoclonal antibodies (mAbs) that recognize glycans are useful tools to assess carbohydrates’ structure and function. We sought to produce IgG mAbs to the human milk oligosaccharide (HMO), lacto-N-fucopentaose III (LNFPIII). LNFPIII contains the Lewisx antigen, which is found on the surface of schistosome parasites. mAbs binding the Lewisx antigen are well-reported in the literature, but mAbs recognizing HMO structures are rare. To generate mAbs, mice were immunized with LNFPIII-DEX (P3DEX) plus CpGs in VacSIM®, a novel vaccine/drug delivery platform. Mice were boosted with LNFPIII-HSA (P3HSA) plus CpGs in Incomplete Freund’s Adjuvant (IFA). Splenocytes from immunized mice were used to generate hybridomas and were screened against LNFPIII conjugates via enzyme-linked immunosorbent assay (ELISA). Three positive hybridomas were expanded, and one hybridoma, producing IgG and IgM antibodies, was cloned via flow cytometry. Clone F1P2H4D8D5 was selected because it produced IgG1 mAbs, but rescreening unexpectedly showed binding to both LNFPIII and lacto-N-neotetraose (LNnT) conjugates. To further assess the specificity of the mAb, we screened it on two glycan microarrays and found no significant binding. This finding suggests that the mAb binds to the acetylphenylenediamine (APD) linker-spacer structure of the conjugate. We present the results herein, suggesting that our new mAb could be a useful probe for conjugates using similar linker spacer structures.
Monoclonal antibodies (mAbs) that recognize glycans are useful tools to assess carbohydrates’ structure and function. We sought to produce IgG mAbs to the human milk oligosaccharide (HMO), lacto-N-fucopentaose III (LNFPIII). LNFPIII contains the Lewisx antigen, which is found on the surface of schistosome parasites. mAbs binding the Lewisx antigen are well-reported in the literature, but mAbs recognizing HMO structures are rare. To generate mAbs, mice were immunized with LNFPIII-DEX (P3DEX) plus CpGs in VacSIM®, a novel vaccine/drug delivery platform. Mice were boosted with LNFPIII-HSA (P3HSA) plus CpGs in Incomplete Freund’s Adjuvant (IFA). Splenocytes from immunized mice were used to generate hybridomas and were screened against LNFPIII conjugates via enzyme-linked immunosorbent assay (ELISA). Three positive hybridomas were expanded, and one hybridoma, producing IgG and IgM antibodies, was cloned via flow cytometry. Clone F1P2H4D8D5 was selected because it produced IgG1 mAbs, but rescreening unexpectedly showed binding to both LNFPIII and lacto-N-neotetraose (LNnT) conjugates. To further assess the specificity of the mAb, we screened it on two glycan microarrays and found no significant binding. This finding suggests that the mAb binds to the acetylphenylenediamine (APD) linker-spacer structure of the conjugate. We present the results herein, suggesting that our new mAb could be a useful probe for conjugates using similar linker spacer structures.
Jessica Ramadhin, Vanessa Silva-Moraes, Thomas Norberg, Donald Harn. Antibodies 2020, 9, 48. https://doi.org/10.3390/antib9030048
