Inherently Reduced Expression of ASC Restricts Caspase-1 Processing in Hepatocytes and Promotes Plasmodium Infection

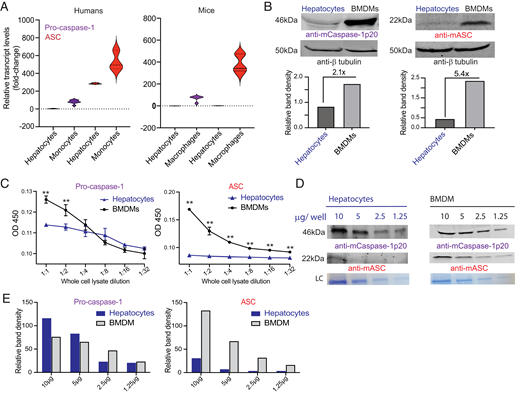
Inflammasome-mediated caspase-1 activation facilitates innate immune control of Plasmodium in the liver, thereby limiting the incidence and severity of clinical malaria. However, caspase-1 processing occurs incompletely in both mouse and human hepatocytes and precludes the generation of mature IL-1β or IL-18, unlike in other cells. Why this is so or how it impacts Plasmodium control in the liver has remained unknown. We show that an inherently reduced expression of the inflammasome adaptor molecule apoptosis-associated specklike protein containing CARD (ASC) is responsible for the incomplete proteolytic processing of caspase-1 in murine hepatocytes. Transgenically enhancing ASC expression in hepatocytes enabled complete caspase-1 processing, enhanced pyroptotic cell death, maturation of the proinflammatory cytokines IL-1β and IL-18 that was otherwise absent, and better overall control of Plasmodium infection in the liver of mice. This, however, impeded the protection offered by live attenuated antimalarial vaccination. Tempering ASC expression in mouse macrophages, on the other hand, resulted in incomplete processing of caspase-1. Our work shows how caspase-1 activation and function in host cells are fundamentally defined by ASC expression and offers a potential new pathway to create better disease and vaccination outcomes by modifying the latter.
Camila Marques-da-Silva, Clyde Schmidt-Silva, Rodrigo P Baptista, Samarchith P Kurup. J Immunol. 2023 Dec 27:ji2300440. doi: 10.4049/jimmunol.2300440. Online ahead of print.
