Focus on Faculty: Courtney Murdock

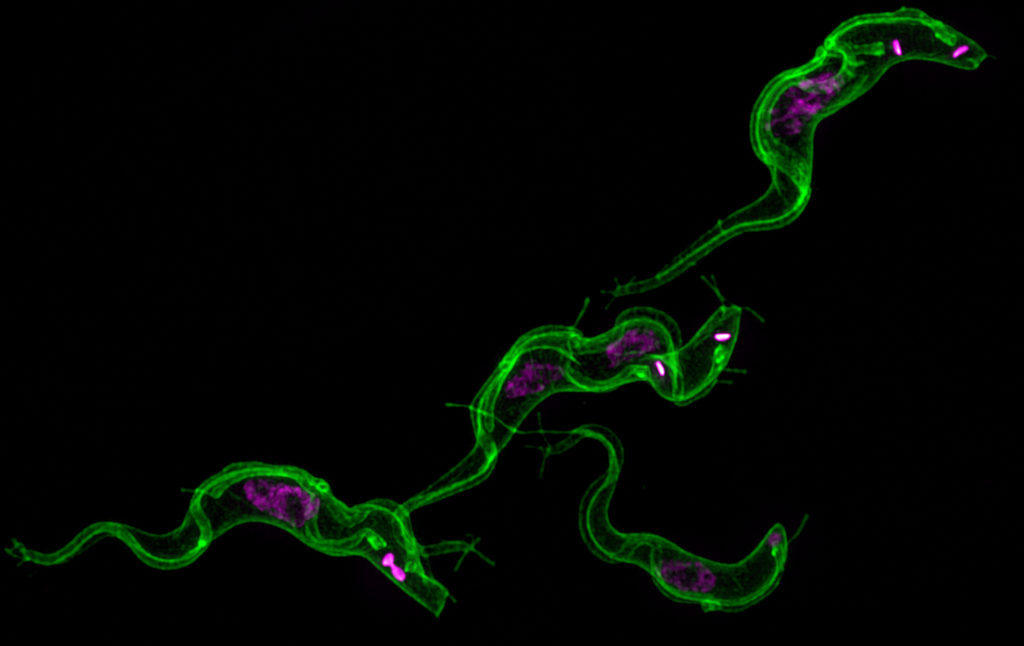
Courtney Murdock, an assistant professor with a joint appointment in the College of Veterinary Medicine, the Odum School of Ecology and CTEGD, studies the transmission of mosquito-borne diseases to inform predictions about disease patterns and interventions to disrupt transmission.
Where did you earn degrees and what are your current responsibilities at UGA?
I earned my Bachelor of Science degree in biology with a minor in Spanish literature at the University of Michigan, where I also earned my Ph.D. in the School of Natural Resources and the Environment. I was a postdoctoral researcher in the departments of biology and entomology at Pennsylvania State University and am currently an assistant professor with a joint appointment in the department of infectious diseases in the UGA College of Veterinary Medicine and the Odum School of Ecology.
When did you come to UGA and what brought you here?
I began my current position at UGA in 2014. I was excited to join the faculty here due to the growing expertise in infectious diseases across campus, having access to excellent colleagues in the College of Veterinary Medicine and the world-renowned Odum School of Ecology, and the plethora of resources available concerning facilities, expertise and support for graduate students.
What are your favorite courses and why?
My favorite courses that I took as an undergraduate and graduate student, and to teach as a professor, are ecology courses. Ecology is a modern science that is the study of the interactions among organisms and the environment. This field of study provides key insights into how the environment shapes interactions among organisms, their abundances, where they live, and our overall impact. Ecological knowledge is crucial for understanding and mitigating some of the biggest problems we will have to contend with in the future—some of which include global climate change, natural catastrophes, food and water scarcity, the evolution of antibiotic resistance, and emerging infectious diseases.
What are some highlights of your career at UGA?
My research on mosquito-borne diseases has been well-supported by agencies such as the National Institutes of Health and National Science Foundation, with total funding exceeding $1.2 million since 2014. These funds have supported laboratory research, as well as fieldwork in the U.S. and the Caribbean. The results of my research have been published in high-quality scientific journals of international standing, and my research findings have been cited nearly 1,000 times.
I also mentor 17 undergraduate students, one D.V.M. student, five Ph.D. students, and two postdoctoral researchers. All of my mentees gain hands-on experience working in an infectious disease system in the lab or field, as well as exposure to a diversity of host-parasite/pathogen systems and projects that are both basic and applied in nature. My students have a strong record of success, with two NSF Graduate Research Fellowships, four travel awards to attend international conferences to present their work, and two awards for presenting research at local venues.
How do you describe the scope and impact of your research or scholarship to people outside of your field?
I am interested in understanding what drives the transmission of mosquito-borne diseases. The mosquito is the deadliest organism on this planet because of the harmful organisms it transmits to humans, wildlife and domestic animals. Many of these diseases cannot be treated with drugs or prevented with vaccines. Thus, only through an understanding of the transmission process will we be able understand when we are at most risk to contract these diseases, predict how current disease distributions might change in the future, and develop interventions that efficiently disrupt transmission.
How does your research or scholarship inspire your teaching, and vice versa?
For me there is quite a bit of cross-talk between my research knowledge and experiences and my teaching. One important goal as an instructor in the sciences is to impart a solid understanding of the scientific process. Many students who take my courses do not necessarily want a career in science. I believe that to be informed citizens, however, they need to be able to think critically about science and its contributions to society. The best way I have found outside of lab sections to impart this knowledge is from drawing on my own research experiences. I also have found that my teaching informs the direction of my research program because it encourages me to think about my research from the perspective of foundational concepts in ecology.
What do you hope students gain from their classroom experience with you?
In general, the learning objectives for my courses include understanding the conceptual foundations of ecology, becoming comfortable understanding and working with scientific data, being familiar with the scientific method, and being able to engage in discussion and make informed decisions about ecological and environmental issues.
On the less concrete side, I want them to wonder at how amazing the natural world is, be curious about it, understand our part and overall impact, and to be more informed, science-literate citizens.
Describe your ideal student.
Here are some characteristics I value in both undergraduate and graduate students that I work with (this is not ranked in any particular order):
- Curiosity—always questioning why and how.
- Self-starter—only you can advocate for your interests and education.
- Life learner—there is no rubric for life; college and graduate school is the perfect place to begin learning how to teach yourself the material you need to know to pass the test, complete course objectives, fulfill job expectations, answer your own questions, etc.
- Positive—this shapes everything, your outlook on life and work, general happiness, interactions with co-workers.
- Hardworking – willing to do what is needed to get the task at hand done.
- Creative – ability to think outside of the box, willingness to explore and adopt concepts from other fields in order to innovate or solve existing problems.
- Team member – working effectively with people with different backgrounds, knowledge, working styles and personalities is a life skill that is beneficial across a diversity of situations and careers.
- Fearless – failure is an opportunity to learn and grow.
- Responsible – this goes beyond just being reliable and detail oriented. It involves taking ownership over success and failure as well as both positive and negative interactions with others.
- Human – have outside interests, be respectful to others, empathize with others.

Favorite place to be/thing to near campus is…
… eating lunch at Cali N Tito’s with colleagues or members of my lab.
Beyond the UGA campus, I like to…
In addition to being a scientist and a professor, I am a mother of two kids, a wife, a daughter, a sister, and a friend. I spend as much time as I can manage with my family and friends outside of work. This involves simple things like going swimming with the kids at the YMCA, going to the local library, going to museums or Lego Land in Atlanta, playing at local parks when the weather is nice, hiking at Fort Yargo State Park or Sandy Creek Nature Center, and occasionally camping in the mountains of Georgia. We also spend a lot of our vacation visiting family in Chicago, Illinois, and Traverse City, Michigan.
Favorite book/movie (and why)?
Favorite nonfiction: “Devil in the White City.” I grew up in the suburbs of Chicago, so it was really insightful and fun to read about how much of the city and world was shaped by the World Fair of 1893. There is also a side story involving a serial killer, which is totally gripping.
Favorite fiction: The “Outlander” series by Diana Gabaldon. These novels are historical fiction, mixed with fantasy and a pinch of romance. The characters are well developed, complex, and the history well researched, so is a perfect storm for losing oneself completely.
The one UGA experience I will always remember will be…
Every year my lab picks a themed costume and dresses up for Halloween. To me this is special, as it is an opportunity for our group to do something fun, wacky and together. Current pictures are on our website: https://www.themurdocklab.com/people.
Is there anything else you’d like to add?
I was an NCAA Division 1 scholar-athlete. I walked on to the University of Michigan softball team as a freshman during my undergraduate career. While I never started, I learned a lot of life skills from this experience that translate to my perspective on life, challenges, teamwork and leadership. I feel like there are stereotypes associated with student-athletes that are oftentimes unwarranted concerning their scholarship, and we should be mindful of this in our interactions with student-athletes in the classroom. I feel that they bring a lot of underappreciated assets to the table.
First published at UGA Today.