The Toxoplasma gondii F-Box Protein L2 Functions as a Repressor of Stage Specific Gene Expression
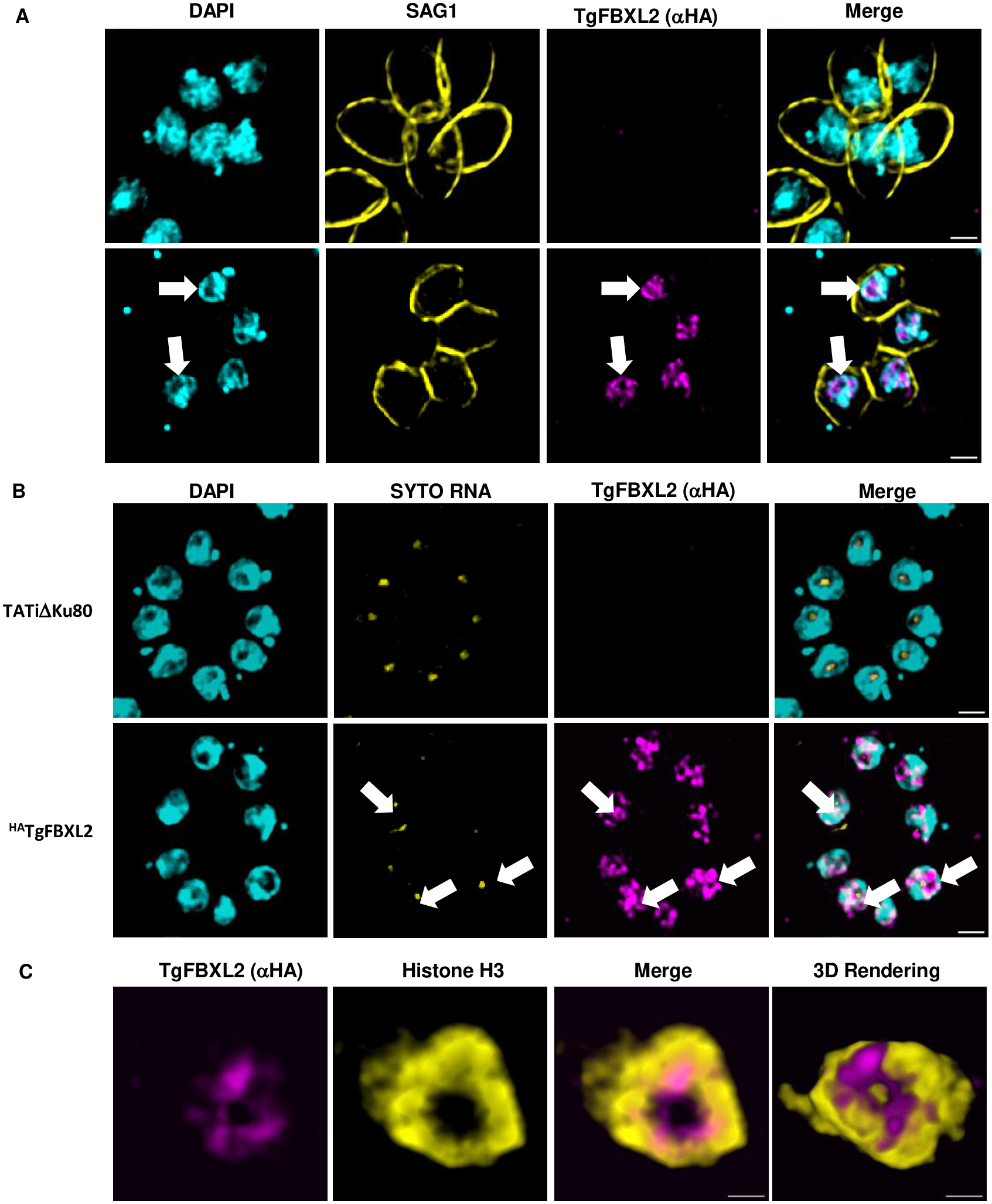
Toxoplasma gondii is a foodborne pathogen that can cause severe and life-threatening infections in fetuses and immunocompromised patients. Felids are its only definitive hosts, and a wide range of animals, including humans, serve as intermediate hosts. When the transmissible bradyzoite stage is orally ingested by felids, they transform into merozoites that expand asexually, ultimately generating millions of gametes for the parasite sexual cycle. However, bradyzoites in intermediate hosts differentiate exclusively to disease-causing tachyzoites, which rapidly disseminate throughout the host. Though tachyzoites are well-studied, the molecular mechanisms governing transitioning between developmental stages are poorly understood. Each parasite stage can be distinguished by a characteristic transcriptional signature, with one signature being repressed during the other stages. Switching between stages require substantial changes in the proteome, which is achieved in part by ubiquitination. F-box proteins mediate protein poly-ubiquitination by recruiting substrates to SKP1, Cullin-1, F-Box protein E3 ubiquitin ligase (SCF-E3) complexes. We have identified an F-box protein named Toxoplasma gondii F-Box Protein L2 (TgFBXL2), which localizes to distinct perinucleolar sites. TgFBXL2 is stably engaged in an SCF-E3 complex that is surprisingly also associated with a COP9 signalosome complex that negatively regulates SCF-E3 function. At the cellular level, TgFBXL2-depleted parasites are severely defective in centrosome replication and daughter cell development. Most remarkable, RNAseq data show that TgFBXL2 conditional depletion induces the expression of stage-specific genes including a a large cohort of genes necessary for sexual commitment. Together, these data suggest that TgFBXL2 is a latent guardian of stage specific gene expression in Toxoplasma and poised to remove conflicting proteins in response to an unknown trigger of development.
Carlos G Baptista, Sarah Hosking, Elisabet Gas-Pascual, Loic Ciampossine, Steven Abel, Mohamed-Ali Hakimi, Victoria Jeffers, Karine Le Roch, Christopher M West, Ira J Blader. PLoS Pathog. 2024 May 30;20(5):e1012269. doi: 10.1371/journal.ppat.1012269.
