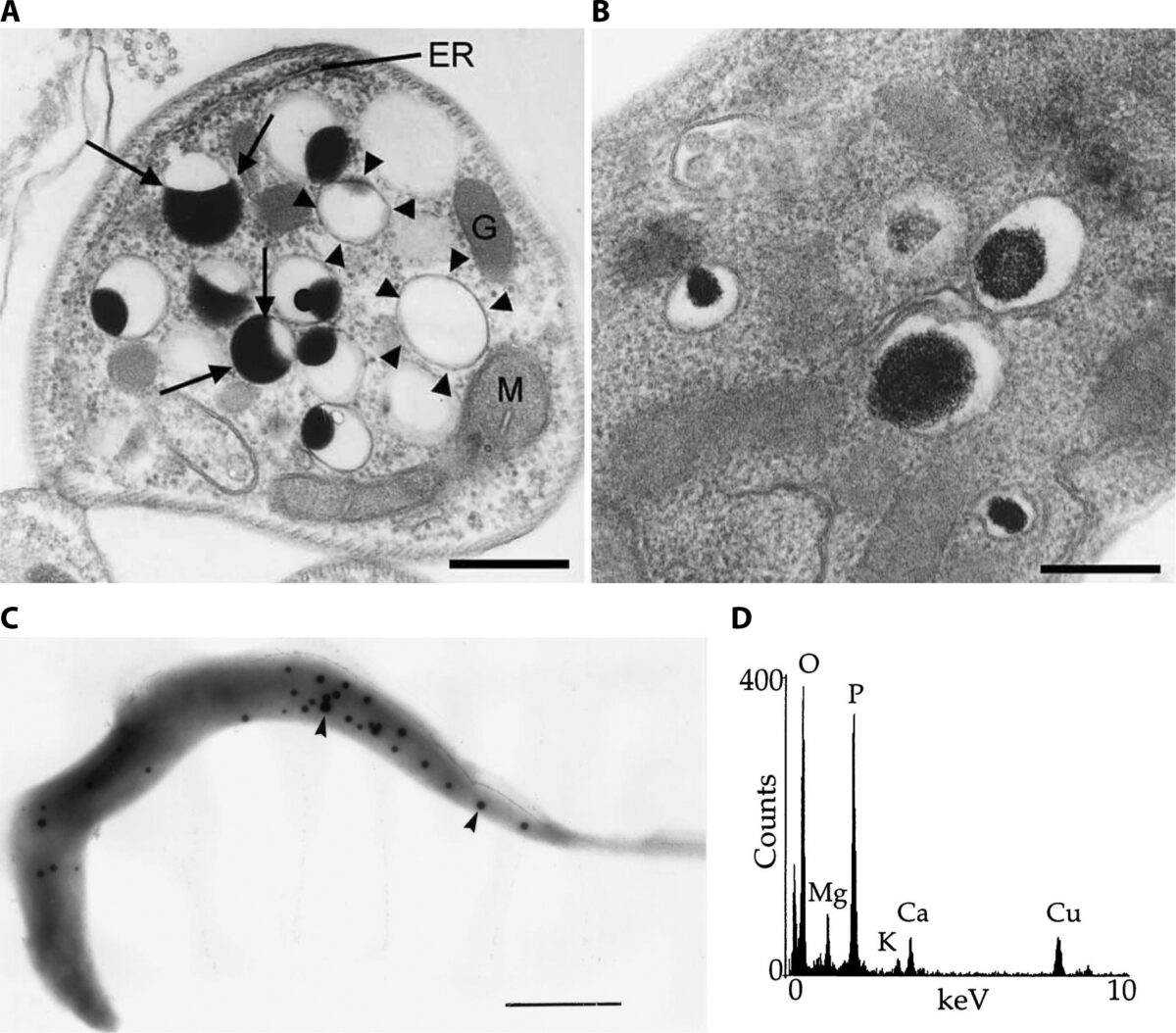
Imaging Ca2+ Signaling in Trypanosoma cruzi with Genetically Encoded Ca2+ Indicators
Genetically encoded calcium indicators (GECIs) are proteins used to monitor calcium ion (Ca2+) changes in living cells. They can be targeted to the cytosol or to organelles for continuous measurements in vivo. In this chapter, we present an overview of the methods that our lab used to detect Ca2+ changes in Trypanosoma cruzi elicited by stimulation …