On the origin and evolution of the mosquito male-determining factor Nix
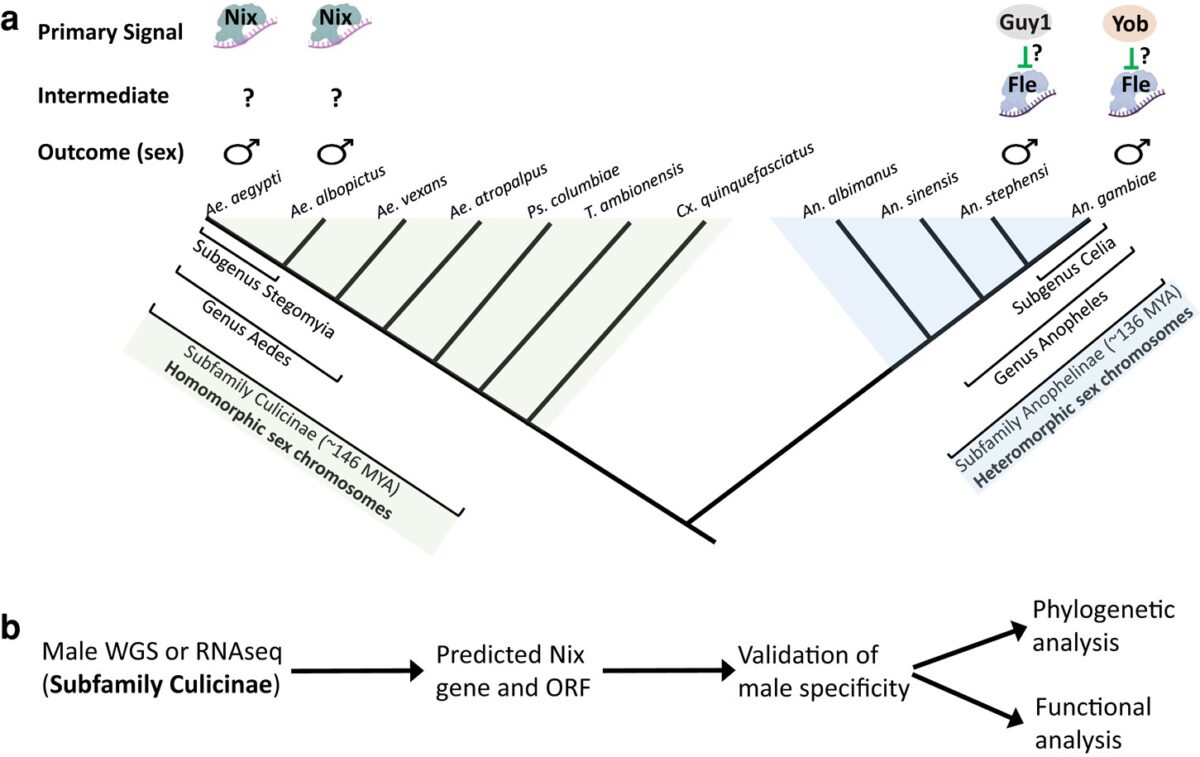
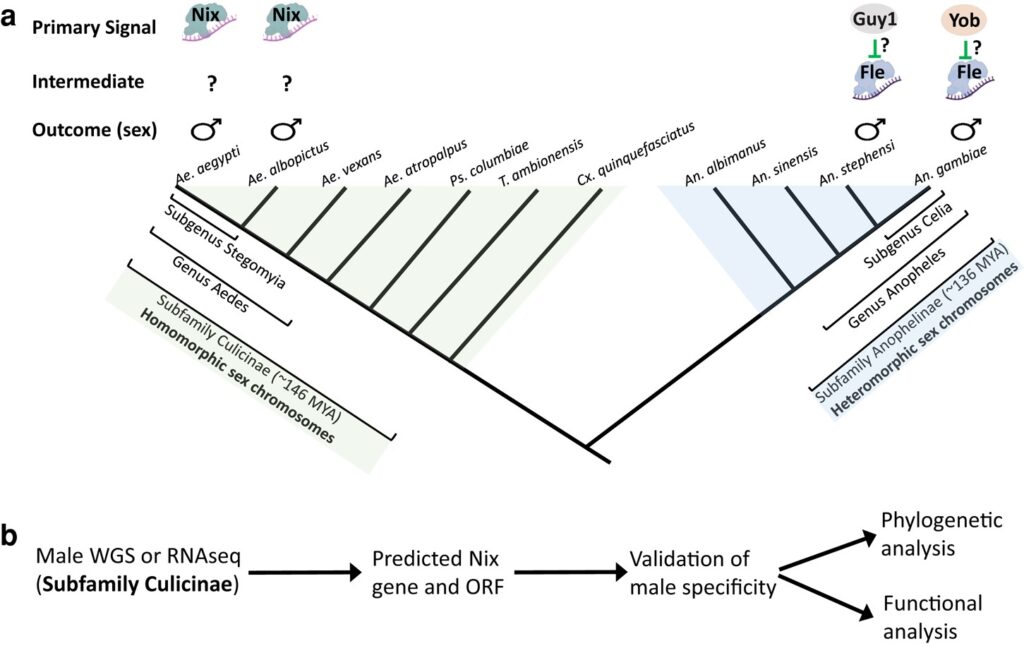
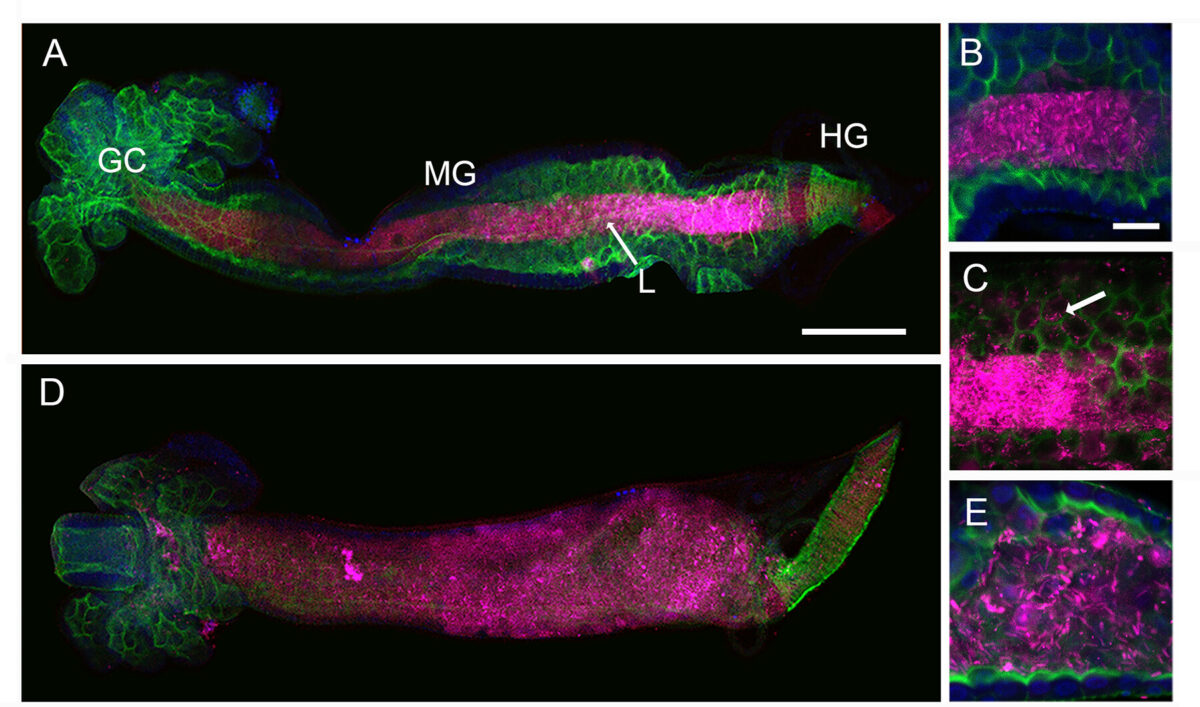
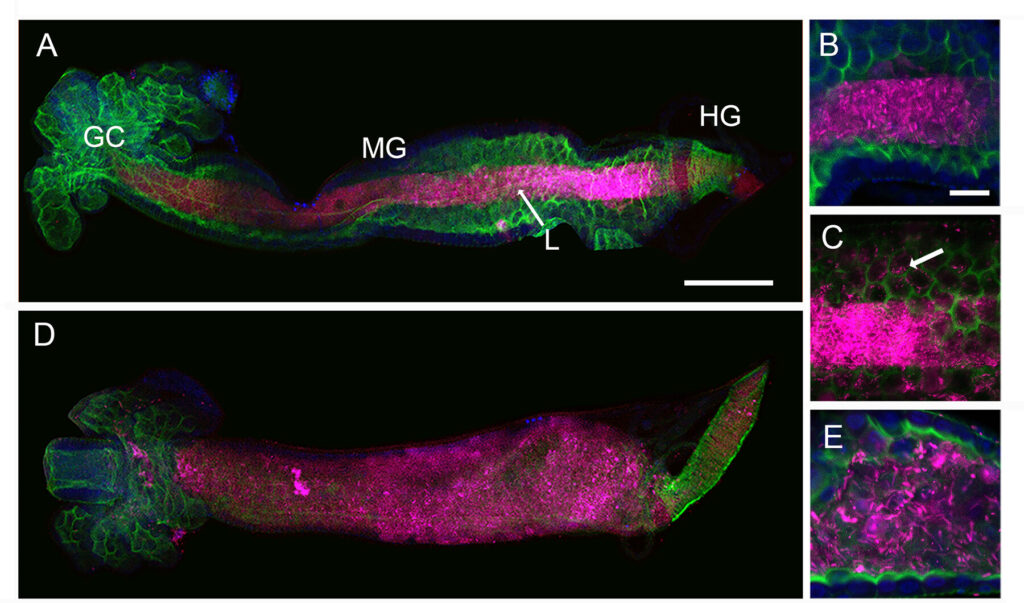
The mosquito family Culicidae is divided into two subfamilies named the Culicinae and Anophelinae. Nix, the dominant male-determining factor, has only been found in the culicines Aedes aegypti and Ae. albopictus, two important arboviral vectors that belong to the subgenus Stegomyia. Here we performed sex-specific whole-genome sequencing and RNAseq of divergent mosquito species and explored additional male-inclusive datasets to investigate the distribution of Nix. Except for the Culex genus, Nix homologs were found in all species surveyed from the Culicinae subfamily, including 12 additional species from three highly divergent tribes comprising 4 genera, suggesting Nix originated at least 133-165 MYA. Heterologous expression of one of three divergent Nix ORFs in Ae. aegypti resulted in partial masculinization of genetic females as evidenced by morphology and doublesex splicing. Phylogenetic analysis suggests Nix is related to femaleless (fle), a recently described intermediate sex-determining factor found exclusively in anopheline mosquitoes. Nix from all species has a conserved structure, including three RNA-recognition motifs (RRMs), as does fle. However, Nix has evolved at a much faster rate than fle. The RRM3 of both Nix and fle are distantly related to the single RRM of a widely distributed and conserved splicing factor transformer-2 (tra2). RRM3-based phylogenetic analysis suggests this domain in Nix and fle may have evolved from tra2 or a tra2-related gene in a common ancestor of mosquitoes. Our results provide insights into the evolution of sex-determination in mosquitoes and will inform broad applications of mosquito-control strategies based on manipulating sex ratios towards the non-biting males.
James K Biedler, Azadeh Aryan, Yumin Qi, Aihua Wang, Ellen O Martinson, Daniel A Hartman, Fan Yang, Atashi Sharma, Katherine S Morton, Mark Potters, Chujia Chen, Stephen L Dobson, Gregory D Ebel, Rebekah C Kading, Sally Paulson, Rui-De Xue, Michael R Strand, Zhijian Tu. Mol Biol Evol. 2023 Dec 21:msad276. doi: 10.1093/molbev/msad276. Online ahead of print.