Visiting Scholar Fellow: Fernando Sanchez-Valdéz
 Dr. Fernando Sanchez-Valdéz, from Salta, Argentina, completed a Ph.D. in Molecular Biology at the Faculty of Pharmacy and Biochemistry at the University of Buenos Aires, Argentina in 2014. After his Ph.D., he completed a postdoctoral fellowship in Dr. Rick Tarleton´s laboratory at University of Georgia. In 2018, he obtained a Research Scientist position in the career pathway of the National Research Council in Argentina (CONICET). Earlier this year, he was awarded a fellowship from the CTEGD-Janssen Visiting Scholars Program, which enabled him to return to the Tarleton Research Group.
Dr. Fernando Sanchez-Valdéz, from Salta, Argentina, completed a Ph.D. in Molecular Biology at the Faculty of Pharmacy and Biochemistry at the University of Buenos Aires, Argentina in 2014. After his Ph.D., he completed a postdoctoral fellowship in Dr. Rick Tarleton´s laboratory at University of Georgia. In 2018, he obtained a Research Scientist position in the career pathway of the National Research Council in Argentina (CONICET). Earlier this year, he was awarded a fellowship from the CTEGD-Janssen Visiting Scholars Program, which enabled him to return to the Tarleton Research Group.
What is your primary research focus? Why are you interested in this subject?
The main focus of my research has been to uncover the mechanism of drug resistance in the Chagas disease agent, Trypanosoma cruzi. The main question we are trying to answer is why the treatment with highly effective drugs like Benznidazole (the current available treatment for Chagas disease) often fails to cure Chagas disease. By combining ex vivo luminescence assays and tissue-clearing techniques we were able to report, for the first time, the presence of dormant non-replicating amastigotes forms in the chronic phase of the disease. Dormant amastigotes were uniquely resistant to extended drug treatment in vivo and in vitro and could re-establish a flourishing infection after treatment interruption. T. cruzi‘s capacity to become dormant makes them transiently drug-resistant, suggesting that this phenomenon accounts for the failure of the otherwise highly active compounds such Benznidazole (Sanchez-Valdéz, et al eLife 2018).
Why did you choose UGA?
I returned to Athens in February 2019 to continue working on the findings we made during my postdoctoral training in the Tarleton Laboratory. I initially decided to come UGA based on a colleague’s recommendations and the fact that Tarleton´s lab is one of the reference centers for Chagas disease research. It’s a really motivating environment to do science since the scientific and technical level here is really high as well as diverse including areas as immunology, drug discovery, genetic manipulation, genomics, diagnostics, etc. Also the amount of resources available is impressive not only from the lab but also from the Biomedical Microscopy Core, Cytometry Shared Resource Laboratory and the animal facility at UGA.
What has been your research project while at UGA?
Currently, we are expanding our knowledge about T. cruzi dormancy and trying to interfere T. cruzi dormancy using new compounds or the conventional drugs but in a different treatment schedule. One of the approaches we are testing now involves the evaluation of drug doses and treatment schemes able to kill dormant parasites. For this purpose, we are optimizing a robust platform to detect low levels of parasites in whole clarified mice organs using light-sheet fluorescent microscopy. This technique will allow us the specific detection of low levels of persistent dormant parasites.
How has the CTEGD-Janssen Visiting Scholar Fellowship and your time at UGA impacted your research and professional goals?
I am so glad about the opportunity to continue working on T. cruzi dormancy with such experienced and renowned scientists and particularly using state-of-the-art microscopy techniques currently unavailable in South America. This experience will definitely have a positive impact on my career development and probably in the Chagas disease research field.


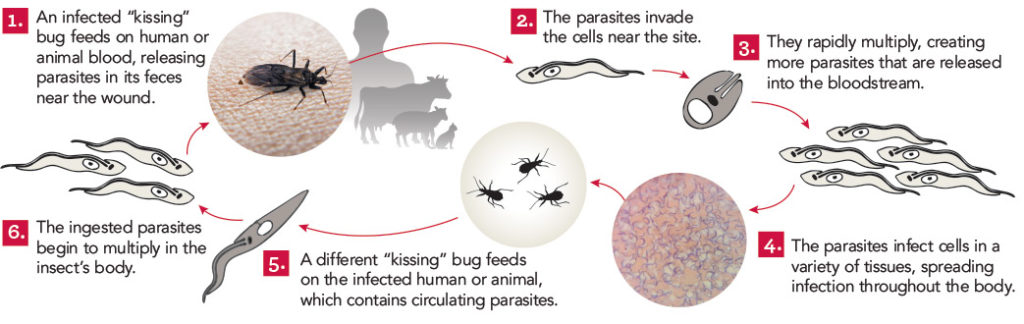


 Athens, Ga. – University of Georgia researchers in collaboration with Anacor Pharmaceuticals have received a $5.3 million grant from the Wellcome Trust to develop a new drug for the treatment of Chagas disease, which they hope will be ready to enter clinical trials by 2016.
Athens, Ga. – University of Georgia researchers in collaboration with Anacor Pharmaceuticals have received a $5.3 million grant from the Wellcome Trust to develop a new drug for the treatment of Chagas disease, which they hope will be ready to enter clinical trials by 2016.
