Immune exhaustion in chronic Chagas disease: Pro-inflammatory and immunomodulatory action of IL-27 in vitro
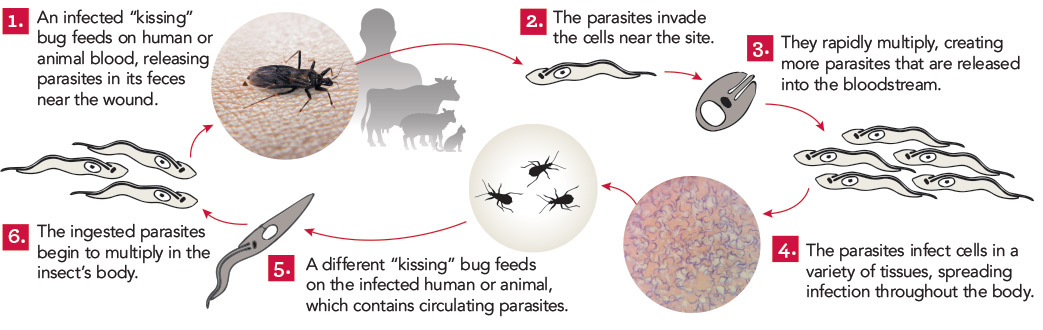
In chronic Chagas disease, Trypanosoma cruzi-specific T-cell function decreases over time, and alterations in the homeostatic IL-7/IL-7R axis are evident, consistent with a process of immune exhaustion. IL-27 is an important immunoregulatory cytokine that shares T-cell signaling with IL-7 and other cytokines of the IL-12 family and might be involved in the transcriptional regulation of T-cell function. Here, we evaluated the expression and function of IL-27R in antigen-experienced T cells from subjects with chronic Chagas disease and assessed whether in vitro treatment with IL-27 and IL-7 might improve T. cruzi-specific polyfunctional T-cell responses. In vitro exposure of PBMCs to T. cruzi induced a downregulation of IL-27R in CD4+ T cells and an upregulation in CD8+ T cells in subjects without heart disease, while IL-27R expression remained unaltered in subjects with more severe clinical stages. The modulation of IL-27R was associated with functional signaling through STAT3 and STAT5 and induction of the downstream genes TBX21, EOMES and CXCL9 in response to IL-27. In vitro treatment of PBMCs with IL-27 and IL-7 improved monofunctional and polyfunctional Th1 responses, accompanied by the induction of IL-10 and Bcl-2 expression in subjects without heart disease but did not improve those in subjects with cardiomyopathy. Our findings support the process of desensitization of the IL-27/IL-27R pathway along with disease severity and that the pro-inflammatory and immunomodulatory mechanisms of IL-27 might be interconnected.
María Ailén Natale, Todd Minning, María Cecilia Albareda, Melisa Daiana Castro Eiro, María Gabriela Álvarez, Bruno Lococo, Gonzalo Cesar, Graciela Bertocchi, María Josefina Elias, María Belén Caputo, Rick Lee Tarleton, Susana Adriana Laucella. PLoS Negl Trop Dis. 2021 Jun 1;15(6):e0009473. doi: 10.1371/journal.pntd.0009473.