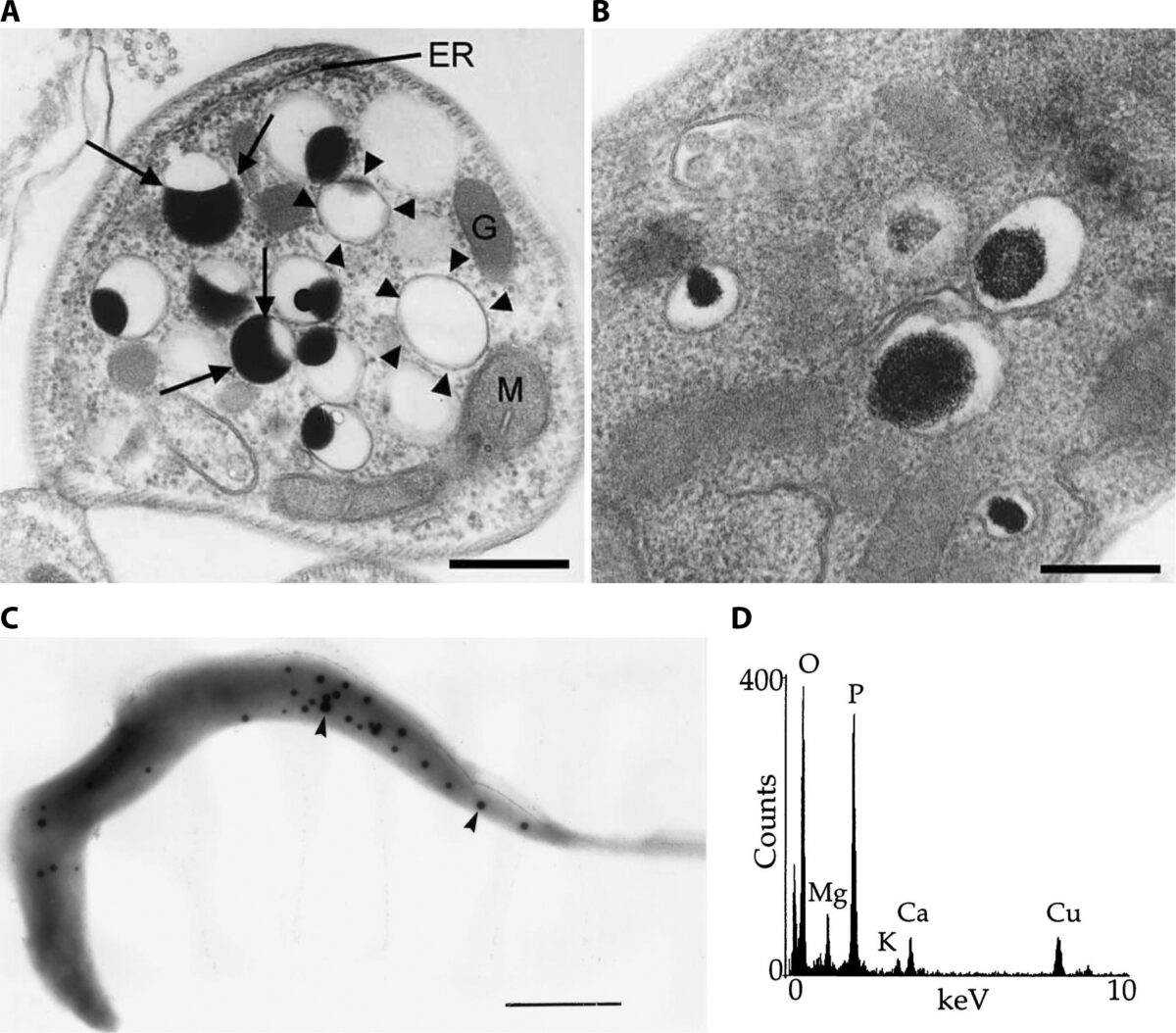
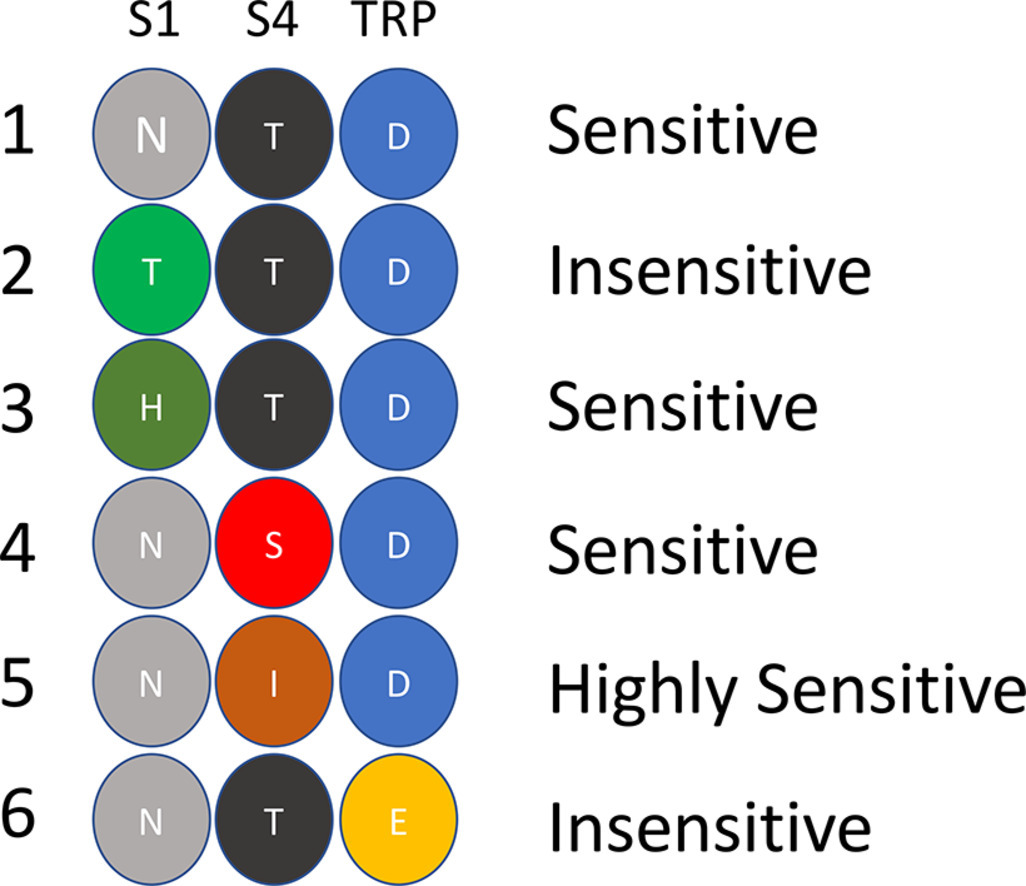
Regulation of Calcium entry by cyclic GMP signaling in Toxoplasma gondii
Ca2+ signaling impacts almost every aspect of cellular life. Ca2+ signals are generated through the opening of ion channels that permit the flow of Ca2+ down an electrochemical gradient. Cytosolic Ca2+ fluctuations can be generated through Ca2+ entry from the extracellular milieu or release from intracellular stores. In Toxoplasma gondii, Ca2+ ions play critical roles in several essential functions for the parasite like invasion of host cells, motility and egress. Plasma membrane Ca2+ entry in T. gondii was previously shown to be activated by cytosolic calcium and inhibited by the voltage-operated Ca2+ channel blocker nifedipine. However, Ca2+ entry in T. gondii did not show the classical characteristics of store regulation. In this work, we characterized the mechanism by which cytosolic Ca2+ regulates plasma membrane Ca2+ entry in extracellular T. gondii tachyzoites loaded with the Ca2+ indicator Fura 2. We compared the inhibition by nifedipine with the effect of the broad spectrum TRP channel inhibitor, anthranilic acid or ACA and we find that both inhibitors act on different Ca2+ entry activities. We demonstrate, using pharmacological and genetic tools, that an intracellular signaling pathway engaging cyclicGMP (cGMP), protein kinase G (PKG), Ca2+ and the phosphatidyl inositol phospholipase C (PI-PLC) affects Ca2+ entry and we present a model for crosstalk between cGMP and cytosolic Ca2+ for the activation of T. gondii‘s lytic cycle traits.
Miryam A Hortua Triana, Karla M Márquez-Nogueras, Mojtaba Sedigh Fazli, Shannon Quinn, Silvia N J Moreno. J Biol Chem. 2024 Feb 19:105771. doi: 10.1016/j.jbc.2024.105771