Trainee Spotlight: Stephen Vella
Stephen Vella is a Ph.D. trainee in Silvia Moreno’s laboratory. He is originally from Indiana where he received his B.S. in microbiology at Indiana University. In his first year at UGA, he was awarded an Excellence in Graduate Recruitment Award and a Provost’s Scholars of Excellence Award Fellowship. He has also been awarded an Outstanding Poster Presentation at the Molecular Parasitology Meeting in 2016. And in 2017, he was awarded a T32 fellowship from CTEGD.
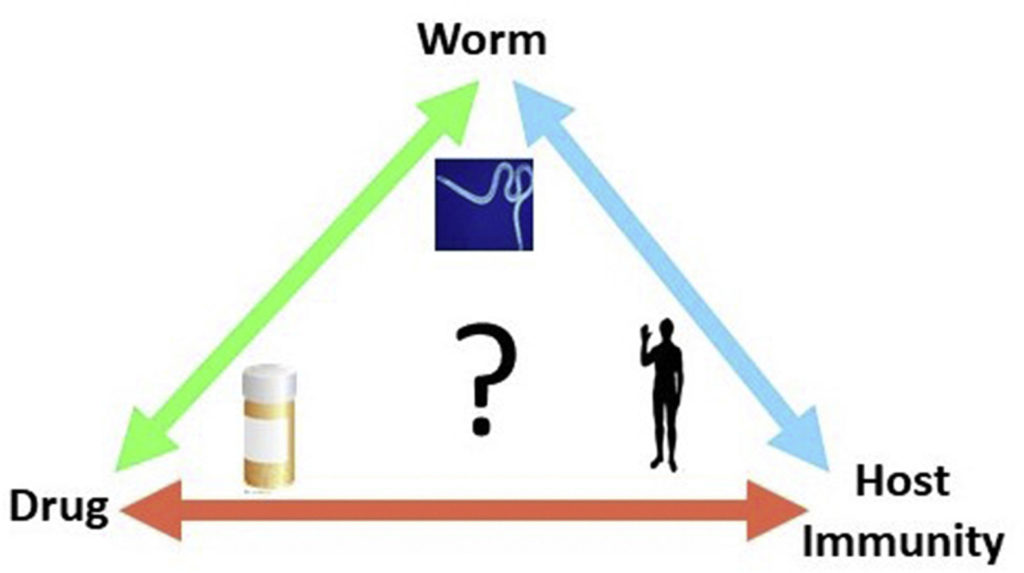
Recognition and killing of Brugia malayi microfilariae by human immune cells is dependent on the parasite sample and is not altered by ivermectin treatment
Abstract
Mass administration of macrocyclic lactones targets the transmission of the causative agents of lymphatic filariasis to their insect vectors by rapidly clearing microfilariae (Mf) from the circulation. It has been proposed that the anti-filarial action of these drugs may be mediated through the host immune system. We recently developed an in vitro assay for monitoring the attachment to and killing of B. malayi Mf by human neutrophils (PMNs) and monocytes (PBMCs), however, the levels of both cell to worm attachment and leukocyte mediated Mf killing varied greatly between individual experiments. To determine whether differences in an individual’s immune cells or the Mf themselves might account for the variability in survival, PMNs and PBMCs were isolated from 12 donors every week for 4 weeks and the cells used for survival assays with a different batch of Mf, thereby keeping donors constant but varying the Mf sample. Results from these experiments indicate that, overall, killing is Mf-rather than donor-dependent. To assess whether ivermectin (IVM) or diethylcarbamazine (DEC) increase killing, Mf were incubated either alone or with immune cells in the presence of IVM or DEC. Neither drug induced a significant difference in the survival of Mf whether cultured with or without cells, with the exception of DEC at 2 h post incubation. In addition, human PBMCs and PMNs were incubated with IVM or DEC for 1 h or 16 h prior to RNA extraction and Illumina sequencing. Although donor-to-donor variation may mask subtle differences in gene expression, principle component analysis of the RNASeq data indicates that there is no significant change in the expression of any genes from the treated cells versus controls. Together these data suggest that IVM and DEC have little direct effect on immune cells involved in the rapid clearance of Mf from the circulation.
Barbara J. Reaves, Connor Wallis, Ciaran J.McCoy, W. Walter Lorenz, Balazs Rada, Adrian J.Wolstenholme. 2018. International Journal for Parasitology: Drugs and Drug Resistance; 6(3): 587-595.
Faculty search underway for Assistant Professor

The Department of Pharmaceutical and Biomedical Sciences in the College of Pharmacy and the Center for Tropical & Emerging Global Diseases at the University of Georgia invites applications for a full-time tenure track position at the level of Assistant Professor.
We seek an individual who will build and maintain a strong extramurally-funded research program focused on parasites that applies cutting-edge approaches in chemical biology, molecular pharmacology, medicinal chemistry, or drug discovery. The successful candidate would build on the research strengths of the Department and the Center for Tropical and Emerging Global Diseases, one of the world’s leading centers for parasite research. A commitment to excellence in teaching at the undergraduate and graduate levels within the College of Pharmacy is also required. The position includes a very competitive salary, excellent laboratory space, and a generous start-up package. Applicants must hold a Ph.D. (or MD) degree in a biomedical, biological, or pharmacological science, have at least two years of postdoctoral training and a strong publication record.
Applicants should submit a cover letter, curriculum vitae, research statement (up to 3 pages) and teaching philosophy. Three confidential letters of recommendation are required, and applicants should provide email addresses for referees in their application. Application materials submitted in other ways will not be accepted. Review of applications will begin on November 15, 2018, and continue until the position is filled. Contact pbsearch@uga.edu with questions.
The College of Pharmacy and the University of Georgia is committed to increasing the diversity of its faculty and students, and sustaining a work and learning environment that is inclusive. The University of Georgia is an Equal Opportunity/Affirmative Action employer. All qualified applicants will receive consideration for employment without regard to race, color, religion, sex, national origin, disability, gender identity, sexual orientation or protected veteran status. Persons needing accommodations or assistance with the accessibility of materials related to this search are encouraged to contact Central HR (facultyjobs@uga.edu). Please do not contact the department or search committee with such requests.
Minimum Qualifications: Applicants must hold a Ph.D. (or MD) degree in a biomedical or biological science, and have at least two years of postdoctoral training.
Applications will be accepted until December 16, 2018.
[button size=’large’ style=” text=’Apply Now’ icon=” icon_color=’#cf0404′ link=’https://www.ugajobsearch.com/postings/33146′ target=’_self’ color=” hover_color=” border_color=” hover_border_color=” background_color=” hover_background_color=” font_style=” font_weight=” text_align=’center’ margin=”]
Inorganic Polyphosphate Interacts with Nucleolar and Glycosomal Proteins in Trypanosomatids
Summary
Inorganic polyphosphate (polyP) is a polymer of three to hundreds of phosphate units bound by high‐energy phosphoanhydride bonds and present from bacteria to humans. Most polyP in trypanosomatids is concentrated in acidocalcisomes, acidic calcium stores that possess a number of pumps, exchangers, and channels, and are important for their survival. In this work, using polyP as bait we identified > 25 putative protein targets in cell lysates of both Trypanosoma cruzi and Trypanosoma brucei. Gene ontology analysis of the binding partners found a significant over‐representation of nucleolar and glycosomal proteins. Using the polyphosphate‐binding domain (PPBD) of Escherichia coliexopolyphosphatase (PPX), we localized long‐chain polyP to the nucleoli and glycosomes of trypanosomes. A competitive assay based on the pre‐incubation of PPBD with exogenous polyP and subsequent immunofluorescence assay of procyclic forms (PCF) of T. brucei showed polyP concentration‐dependent and chain length‐dependent decrease in the fluorescence signal. Subcellular fractionation experiments confirmed the presence of polyP in glycosomes of T. brucei PCF. Targeting of yeast PPX to the glycosomes of PCF resulted in polyP hydrolysis, alteration in their glycolytic flux and increase in their susceptibility to oxidative stress.
Raquel S. Negreiros, Noelia Lander, Guozhong Huang, Ciro D. Cordeiro, Stephanie A. Smith, James H. Morrissey, Roberto Docampo. 2018. Molecular Microbiology; 110(6):973-994. https://doi.org/10.1111/mmi.14131
Calcium-sensitive pyruvate dehydrogenase phosphatase is required for energy metabolism, growth, differentiation, and infectivity of Trypanosoma cruzi
Abstract
In vertebrate cells, mitochondrial Ca2+ uptake by the mitochondrial calcium uniporter (MCU) leads to Ca2+-mediated stimulation of an intramitochondrial pyruvate dehydrogenase phosphatase (PDP). This enzyme dephosphorylates serine residues in the E1α subunit of pyruvate dehydrogenase (PDH), thereby activating PDH and resulting in increased ATP production. Although a phosphorylation–dephosphorylation cycle for the E1α subunit of PDH from non-vertebrate organisms has been described, the Ca2+-mediated PDP activation has not been studied. In this work we investigated the Ca2+ sensitivity of two recombinant PDPs from the protozoan human parasites Trypanosoma cruzi (TcPDP) and Trypanosoma brucei (TbPDP) and generated a TcPDP-KO cell line to establish TcPDP’s role in cell bioenergetics and survival. Moreover, the mitochondrial localization of the TcPDP was studied by CRISPR/Cas9-mediated endogenous tagging. Our results indicate that TcPDP and TbPDP both are Ca2+-sensitive phosphatases. Of note, TcPDP-KO epimastigotes exhibited increased levels of phosphorylated TcPDH, slower growth and lower oxygen consumption rates than control cells, an increased AMP:ATP ratio and autophagy under starvation conditions, and reduced differentiation into infective metacyclic forms. Furthermore, TcPDP-KO trypomastigotes were impaired in infecting culture host cells. We conclude that TcPDP is a Ca2+-stimulated mitochondrial phosphatase that dephosphorylates TcPDH and is required for normal growth, differentiation, infectivity and energy metabolism in T. cruzi. Our results support the view that one of the main roles of the MCU is linked to the regulation of intramitochondrial dehydrogenases.
Noelia Lander, Miguel A. Chiurillo, Mayara S. Bertolini, Melissa Storey, Anibal E. Vercesi and Roberto Docampo. 2018. Journal of Biological Chemistry; 293(45):17402-17417. doi: 10.1074/jbc.RA118.004498
Trainee Spotlight: Josh Butler

New T32 trainee Josh Butler is a third year Ph.D. student in Belen Cassera‘s laboratory. He is from Front Royal, Virginia and completed his B.S. in chemistry at James Madison University in Harrisonburg, Virginia.
Butler decided to pursue his graduate degree at the University of Georgia because of the Integrate Life Sciences program which offers the opportunity to explore a range of research topics. The same interdisciplinary aspect is what he found appealing about the Center for Tropical and Emerging Global Diseases and ultimately why he joined a lab within this department.
“There is no shortage of resources here, ranging from state of the art instrumentation and core facilities to people that are willing to mentor and train successful scientists,” said Butler. “Coming from a smaller institution, I had never really seen anything to this scale and I knew it was something I wanted to experience and become a part of.”
Research Focus
Broadly, Butler’s research is focused on antimalarial drug discovery. More specifically, he is using antimalarial natural products as tools to discover novel drug targets in the malaria parasite Plasmodium falciparum.
Nearly 220 million people have malaria, and it kills nearly half a million people each year. Plasmodium falciparum causes the most severe forms of malaria, such as cerebral malaria, which can lead to brain damage, coma, and death, and placental malaria, which can be life-threatening to both mother and fetus.
“I chose this research because not only does it contribute positively to the global campaign of malaria eradication, but from a training standpoint it would also provide a solid foundation for a career further researching and developing antimicrobial therapies in general.”
Capstone Experience
Each T32 trainee is provided with the opportunity to pursue a capstone experience. Butler hopes to do an internship with a pharmaceutical industry research group that is actively performing anti-parasitic research to experience how the type research he does as a graduate student can translate outside the realm of academia.
“Private-public collaboration in malaria research has really driven drug discovery research in a positive direction and I would like the opportunity to experience that first hand and develop acumen to engage in that type of research in the next stage of my career.”
Future Career Goals
“I would like to continue working in a field of scientific research which can positively impact people’s lives, whether it be through a biomedical or biotechnical avenue.”
Advice for Aspiring Scientists
“Don’t be afraid to fail or be wrong. Learn from it and use it to keep pushing forward. Try to find positives in the negatives.”
Support trainees like Josh by giving today to the Center for Tropical & Emerging Global Diseases.
[button size=’large’ style=” text=’Give Online’ icon=” icon_color=’#b80d32′ link=’https://ctegd.uga.edu/give/’ target=’_self’ color=” hover_color=” border_color=” hover_border_color=” background_color=” hover_background_color=” font_style=” font_weight=” text_align=’center’ margin=”]
The Mitochondrial Ca2+ Uniporter Complex (MCUC) of Trypanosoma brucei Is a Hetero-oligomer That Contains Novel Subunits Essential for Ca2+ Uptake
ABSTRACT
The mitochondrial calcium uniporter complex (MCUC) is a highly selective channel that conducts calcium ions across the organelle inner membrane. We previously characterized Trypanosoma brucei’s MCU (TbMCU) as an essential component of the MCUC required for parasite viability and infectivity. In this study, we characterize its paralog T. brucei MCUb (TbMCUb) and report the identification of two novel components of the complex that we named TbMCUc and TbMCUd. These new MCUC proteins are unique and conserved only in trypanosomatids. In situ tagging and immunofluorescence microscopy revealed that they colocalize with TbMCU and TbMCUb to the mitochondria of T. brucei. Blue Native PAGE and immunodetection analyses indicated that the MCUC proteins exist in a large protein complex with a molecular weight of approximately 380 kDa. RNA interference (RNAi) or overexpression of the TbMCUc and TbMCUd genes significantly reduced or enhanced mitochondrial Ca2+uptake in T. brucei, respectively, without affecting the mitochondrial membrane potential, indicating that they are essential components of the MCUC of this parasite. The specific interactions of TbMCU with TbMCUb, TbMCUc, or TbMCUd were confirmed by coimmunoprecipitation and split-ubiquitin membrane-based yeast two-hybrid (MYTH) assays. Furthermore, combining mutagenesis analysis with MYTH assays revealed that transmembrane helices (TMHs) were determinant of the interactions between TbMCUC subunits. In summary, our study has identified two novel essential components of the MCUC of T. brucei and defined their direct physical interactions with the other subunits that result in a hetero-oligomeric MCUC.
Guozhong Huang, Roberto Docampo. 2018. Molecular Biology and Physiology. DOI: 10.1128/mBio.01700-18
CRISPR/Cas9 Gene Editing to Make Conditional Mutants of Human Malaria Parasite P. falciparum
ABSTRACT
Malaria is a significant cause of morbidity and mortality worldwide. This disease, which primarily affects those living in tropical and subtropical regions, is caused by infection with Plasmodium parasites. The development of more effective drugs to combat malaria can be accelerated by improving our understanding of the biology of this complex parasite. Genetic manipulation of these parasites is key to understanding their biology; however, historically the genome of P. falciparum has been difficult to manipulate. Recently, CRISPR/Cas9 genome editing has been utilized in malaria parasites, allowing for easier protein tagging, generation of conditional protein knockdowns, and deletion of genes. CRISPR/Cas9 genome editing has proven to be a powerful tool for advancing the field of malaria research. Here, we describe a CRISPR/Cas9 method for generating glmS-based conditional knockdown mutants in P. falciparum. This method is highly adaptable to other types of genetic manipulations, including protein tagging and gene knockouts.
Kudyba, H. M., Cobb, D. W., Florentin, A., Krakowiak, M., Muralidharan, V. 2018. J. Vis. Exp. (139), e57747, doi:10.3791/57747
Steve Hajduk to give keynote address at Molecular Parasitology Meeting

Dr. Stephen Hajduk is an Emeritus Professor at the University of Georgia, USA. He has had a long and distinguished career making significant contributions to our understanding of the underlying mechanisms and function of gene expression, RNA editing, human innate immunity to trypanosome infection and the role of membrane nanotubes and extracellular vesicles (EVs) in communication between trypanosomes and with host cells.
He will give the keynote lecture entitled “The Hidden Life of African Trypanosomes” during the 29th Annual Molecular Parasitology Meeting which is currently being held at the Marine Biological Laboratory at Woods Hole, MA. The address will be on Wednesday, September 12 at 5:30 pm in the Lillie Auditorium.


