Dennis Kyle & CTEGD featured in Georgia Trend’s Article

Director Dennis Kyle was interviewed about CTEGD and his research as part of Georgia Trend’s feature article “Moving Innovation Beyond the Walls”. Read the article now.

Director Dennis Kyle was interviewed about CTEGD and his research as part of Georgia Trend’s feature article “Moving Innovation Beyond the Walls”. Read the article now.
Ingestion of an additional blood meal(s) by a hematophagic insect can accelerate development of several vector-borne parasites and pathogens. Most studies, however, offer blood from the same vertebrate host species as the original challenge (for e.g., human for primary and additional blood meals). Here, we show a second blood meal from bovine and canine hosts can also enhance sporozoite migration in Anopheles stephensi mosquitoes infected with the human- and rodent-restricted Plasmodium falciparum and P. berghei, respectively. The extrinsic incubation period (time to sporozoite appearance in salivary glands) showed more consistent reductions with blood from human and bovine donors than canine blood, although the latter’s effect may be confounded by the toxicity, albeit non-specific, associated with the anticoagulant used to collect whole blood from donors. The complex patterns of enhancement highlight the limitations of a laboratory system but are nonetheless reminiscent of parasite host-specificity and mosquito adaptations, and the genetic predisposition of An. stephensi for bovine blood. We suggest that in natural settings, a blood meal from any vertebrate host could accentuate the risk of human infections by P. falciparum: targeting vectors that also feed on animals, via endectocides for instance, may reduce the number of malaria-infected mosquitoes and thus directly lower residual transmission. Since endectocides also benefit animal health, our results underscore the utility of the One Health framework, which postulates that human health and well-being is interconnected with that of animals. We posit this framework will be further validated if our observations also apply to other vector-borne diseases which together are responsible for some of the highest rates of morbidity and mortality in socio-economically disadvantaged populations.
Ashutosh K Pathak, Justine C Shiau, Rafael C S Freitas, Dennis E Kyle. One Health. 2023 Jun 9:17:100582. doi: 10.1016/j.onehlt.2023.100582. eCollection 2023 Dec.
My name is Victoria Mendiola and I am a PhD candidate in Dennis Kyle’s lab studying drug-induced dormancy in Plasmodium falciparum, the parasite responsible for malaria. I have been at UGA for four years but originally received my BSc in Biology and MSc of Integrative Biology from Kennesaw State University in Kennesaw, GA.
My interest in infectious diseases stems from an NSF REU research internship where I was first introduced to the complexities of parasite-host interactions on an organismal level by studying hookworm infections in South American fur seals (SAFS) in the Gottdenker Lab at UGA’s College of Veterinary Medicine.
During my REU, I fell in love with Athens and the scientific community in the area but the large number of tropical disease parasitologists solidified my reason for choosing UGA to continue my studies.
My doctoral research focuses on developing novel high-content imaging assays to incorporate Artemisinin-induced dormant Plasmodium falciparum recovery into the current understanding of drug treatment, therapeutics, and prevention. Of the species of Plasmodium that infect humans, P. falciparum is the deadliest and, unfortunately, is becoming resistant to current treatment options.
In August 2023, I received the CTEGD Training in Tropical and Emerging Global Diseases fellowship. In addition to providing up to two years of funding, there is also the opportunity for a capstone experience. I plan to use the capstone project opportunity to gain essential in-field, on-site training to complement my current wet lab skillset.
My long-term career goal is to utilize my diverse training in physiology, developmental biology, cellular biology, and infectious diseases to design, optimize, and implement phenotypic and behavioral assays in the context of drug discovery and parasite homeostasis.
For students who are interested in joining the Center for Tropical and Emerging Global Diseases, I suggest they take every opportunity to talk to other researchers in and out of their field and organism of study. The sense of community within the CTEGD is unparalleled and should be utilized at every given opportunity. The friends I have made in and outside of the lab is one of my favorite things about being here at UGA (but the local festivals are really fun too).
Support trainees like Victoria by giving today to the Center for Tropical & Emerging Global Diseases.
Mosquitoes shift from detritus-feeding larvae to blood-feeding adults that can vector pathogens to humans and other vertebrates. The sugar and blood meals adults consume are rich in carbohydrates and protein but are deficient in other nutrients including B vitamins. Facultatively hematophagous insects like mosquitoes have been hypothesized to avoid B vitamin deficiencies by carryover of resources from the larval stage. However, prior experimental studies have also used adults with a gut microbiota that could provision B vitamins. Here, we used Aedes aegypti, which is the primary vector of dengue virus (DENV), to ask if carryover effects enable normal function in adults with no microbiota. We show that adults with no gut microbiota produce fewer eggs, live longer with lower metabolic rates, and exhibit reduced DENV vector competence but are rescued by provisioning B vitamins or recolonizing the gut with B vitamin autotrophs. We conclude carryover effects do not enable normal function.
Ruby E Harrison, Xiushuai Yang, Jai Hoon Eum, Vincent G Martinson, Xiaoyi Dou, Luca Valzania, Yin Wang, Bret M Boyd, Mark R Brown, Michael R Strand. Commun Biol. 2023 Nov 13;6(1):1154. doi: 10.1038/s42003-023-05545-z.
The Eukaryotic Pathogen, Vector and Host Informatics Resource (VEuPathDB, https://veupathdb.org) is a Bioinformatics Resource Center funded by the National Institutes of Health with additional funding from the Wellcome Trust. VEuPathDB supports >600 organisms that comprise invertebrate vectors, eukaryotic pathogens (protists and fungi) and relevant free-living or non-pathogenic species or hosts. Since 2004, VEuPathDB has analyzed omics data from the public domain using contemporary bioinformatic workflows, including orthology predictions via OrthoMCL, and integrated the analysis results with analysis tools, visualizations, and advanced search capabilities. The unique data mining platform coupled with >3000 pre-analyzed data sets facilitates the exploration of pertinent omics data in support of hypothesis driven research. Comparisons are easily made across data sets, data types and organisms. A Galaxy workspace offers the opportunity for the analysis of private large-scale datasets and for porting to VEuPathDB for comparisons with integrated data. The MapVEu tool provides a platform for exploration of spatially resolved data such as vector surveillance and insecticide resistance monitoring. To address the growing body of omics data and advances in laboratory techniques, VEuPathDB has added several new data types, searches and features, improved the Galaxy workspace environment, redesigned the MapVEu interface and updated the infrastructure to accommodate these changes.
Jorge Alvarez-Jarreta, et al. Nucleic Acids Res. 2023 Nov 11:gkad1003. doi: 10.1093/nar/gkad1003
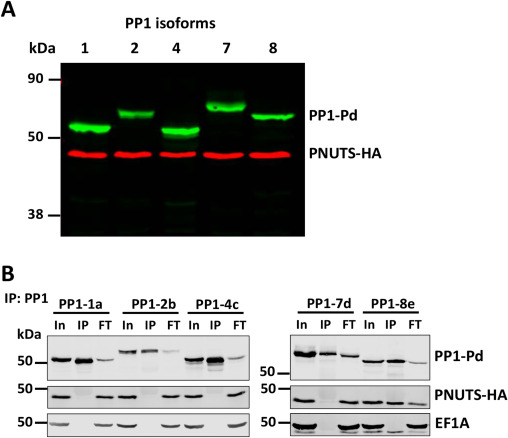
PP1 phosphatases associate with specific regulatory subunits to achieve, among other functions, substrate selectivity. Among the eight PP1 isotypes in Leishmania, PP1-8e associates with the regulatory protein PNUTS along with the structural factors JBP3 and Wdr82 in the PJW/PP1 complex that modulates RNA polymerase II (Pol II) phosphorylation and transcription termination. Little is known regarding interactions involved in PJW/PP1 complex formation, including how PP1-8e is the selective isotype associated with PNUTS. Here, we show that PNUTS uses an established RVxF-ΦΦ-F motif to bind the PP1 catalytic domain with similar interfacial interactions as mammalian PP1-PNUTS and non-canonical motifs. These atypical interactions involve residues within the PP1-8e catalytic domain and N- and C-terminus for isoform specific regulator binding. This work advances our understanding of PP1 isoform selectivity and reveals key roles of PP1 residues in regulator binding. We also explore the role of PNUTS as a scaffold protein for the complex by identifying the C-terminal region involved in binding JBP3 and Wdr82, and impact of PNUTS on the stability of complex components and function in Pol II transcription in vivo. Taken together, these studies provide a potential mechanism where multiple motifs within PNUTS are used combinatorially to tune binding affinity to PP1, and the C-termini for independent binding of JBP3 and Wdr82, in the Leishmania PJW/PP1 complex. Overall, our data provide insights in the formation of the PJW/PP1 complex involved in regulating Pol II transcription in divergent protozoans where little is understood.
Yang Zhang, Robert Sabatini. J Biol Chem. 2023 Nov 3:105432. doi: 10.1016/j.jbc.2023.105432
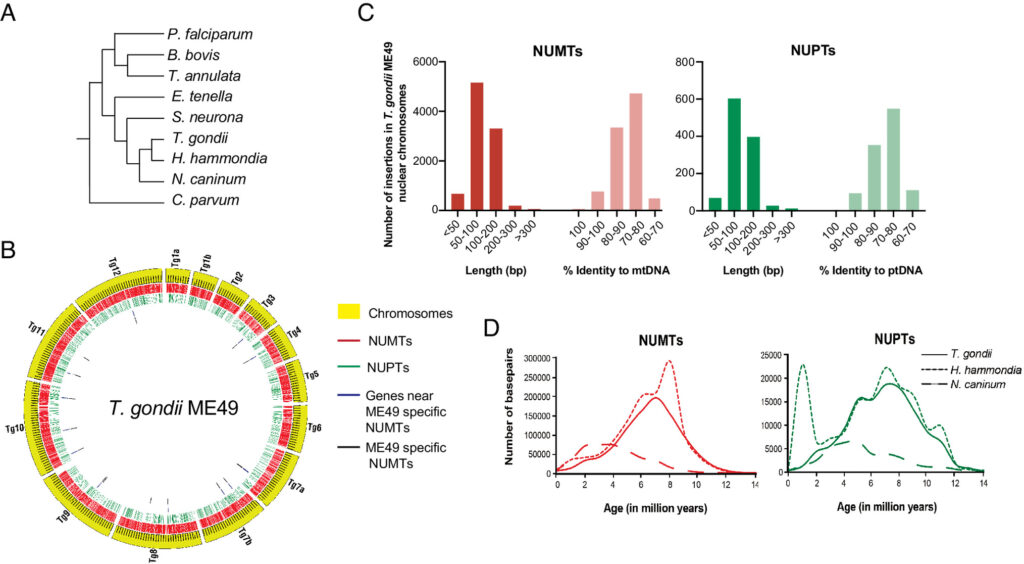
Toxoplasma gondii is a zoonotic protist pathogen that infects up to one third of the human population. This apicomplexan parasite contains three genome sequences: nuclear (65 Mb); plastid organellar, ptDNA (35 kb); and mitochondrial organellar, mtDNA (5.9 kb of non-repetitive sequence). We find that the nuclear genome contains a significant amount of NUMTs (nuclear integrants of mitochondrial DNA) and NUPTs (nuclear integrants of plastid DNA) that are continuously acquired and represent a significant source of intraspecific genetic variation. NUOT (nuclear DNA of organellar origin) accretion has generated 1.6% of the extant T. gondii ME49 nuclear genome-the highest fraction ever reported in any organism. NUOTs are primarily found in organisms that retain the non-homologous end-joining repair pathway. Significant movement of organellar DNA was experimentally captured via amplicon sequencing of a CRISPR-induced double-strand break in non-homologous end-joining repair competent, but not ku80 mutant, Toxoplasma parasites. Comparisons with Neospora caninum, a species that diverged from Toxoplasma ~28 mya, revealed that the movement and fixation of five NUMTs predates the split of the two genera. This unexpected level of NUMT conservation suggests evolutionary constraint for cellular function. Most NUMT insertions reside within (60%) or nearby genes (23% within 1.5 kb), and reporter assays indicate that some NUMTs have the ability to function as cis-regulatory elements modulating gene expression. Together, these findings portray a role for organellar sequence insertion in dynamically shaping the genomic architecture and likely contributing to adaptation and phenotypic changes in this important human pathogen.
Sivaranjani Namasivayam, Cheng Sun, Assiatu B Bah, Jenna Oberstaller, Edwin Pierre-Louis, Ronald Drew Etheridge, Cedric Feschotte, Ellen J Pritham, Jessica C Kissinger. Proc Natl Acad Sci U S A. 2023 Nov 7;120(45):e2308569120. doi: 10.1073/pnas.2308569120.
Mosquitoes including Aedes aegypti are human disease vectors because females must blood feed to produce and lay eggs. Blood feeding triggers insulin-insulin growth factor signaling (IIS) which regulates several physiological processes required for egg development. A. aegypti encodes 8 insulin-like peptides (ILPs) and one insulin-like receptor (IR) plus ovary ecdysteroidogenic hormone (OEH) that also activates IIS through the OEH receptor (OEHR). In this study, we assessed the expression of A. aegypti ILPs and OEH during a gonadotropic cycle and produced each that were functionally characterized to further understand their roles in regulating egg formation. All A. aegypti ILPs and OEH were expressed during a gonadotropic cycle. Five ILPs (1, 3, 4, 7, 8) and OEH were specifically expressed in the head, while antibodies to ILP3 and OEH indicated each was released after blood feeding from ventricular axons that terminate on the anterior midgut. A subset of ILP family members and OEH stimulated nutrient storage in previtellogenic females before blood feeding, whereas most IIS-dependent processes after blood feeding were activated by one or more of the brain-specific ILPs and/or OEH. ILPs and OEH with different biological activities also exhibited differences in IIS as measured by phosphorylation of the IR, phosphoinositide 3-kinase/Akt kinase (AKT) and mitogen-activated protein kinase/extracellular signal-regulated kinase (ERK). Altogether, our results provide the first results that compare the functional activities of all ILP family members and OEH produced by an insect.
Kangkang Chen, Xiaoyi Dou, Jai Hoon Eum, Ruby A Harrison, Mark R Brown, Michael R Strand. Insect Biochem Mol Biol. 2023 Oct 30:104028. doi: 10.1016/j.ibmb.2023.104028.
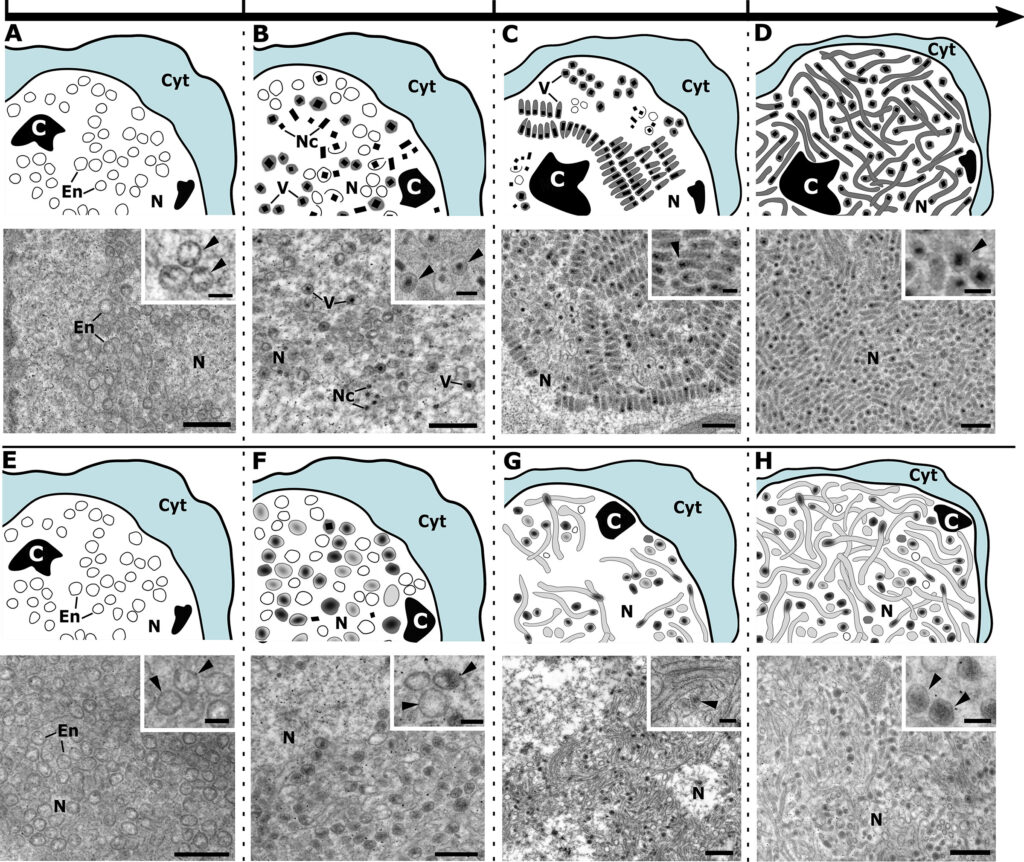
Bracoviruses (BVs) are endogenized nudiviruses in parasitoid wasps of the microgastroid complex (order Hymenoptera: Family Braconidae). BVs produce replication-defective virions that adult female wasps use to transfer DNAs encoding virulence genes to parasitized hosts. Some BV genes are shared with nudiviruses and baculoviruses with studies of the latter providing insights on function, whereas other genes are only known from nudiviruses or other BVs which provide no functional insights. A proteomic analysis of Microplitis demolitor bracovirus (MdBV) virions recently identified 16 genes encoding nucleocapsid components. In this study, we further characterized most of these genes. Some nucleocapsid genes exhibited early or intermediate expression profiles, while others exhibited late expression profiles. RNA interference (RNAi) assays together with transmission electron microscopy indicated vp39, HzNVorf9-like2, HzNVorf93-like, HzNVorf106-like, HzNVorf118-like, and 27b are required to produce capsids with a normal barrel-shaped morphology. RNAi knockdown of vlf-1a, vlf-1b-1, vlf-1b-2, int-1, and p6.9-1 did not alter the formation of barrel-shaped capsids but each reduced processing of amplified proviral segments and DNA packaging as evidenced by the formation of electron translucent capsids. All of the genes required for normal capsid assembly were also required for proviral segment processing and DNA packaging. Collectively, our results deorphanize several BV genes with previously unknown roles in virion morphogenesis.IMPORTANCEUnderstanding how bracoviruses (BVs) function in wasps is of broad interest in the study of virus evolution. This study characterizes most of the Microplitis demolitor bracovirus (MdBV) genes whose products are nucleocapsid components. Results indicate several genes unknown outside of nudiviruses and BVs are essential for normal capsid assembly. Results also indicate most MdBV tyrosine recombinase family members and the DNA binding protein p6.9-1 are required for DNA processing and packaging into nucleocapsids.
Ange Lorenzi, Michael J Arvin, Gaelen R Burke, Michael R Strand. J Virol. 2023 Oct 25:e0081723. doi: 10.1128/jvi.00817-23.
by Amy Horton

New compound targets P. vivax, source of recent U.S. infections
Two University of Georgia researchers have been awarded approximately $770,000 from the Global Health Initiative Technology (GHIT) Fund to develop a new drug to kill the dormant liver stages of Plasmodium vivax, the most widespread of the malaria parasites. This amount is part of a total of JPY 334,238,778 awarded by the GHIT Fund to a partnership consisting of UGA, Medicine for Malaria Venture and Mitsubishi Tanabe Pharma Corporation.
“P. vivax often persists in the liver of patients, causing a relapse infection following treatment of the symptomatic blood infection,” said Steven Maher, associate research scientist in the Office of Research’s Center for Tropical and Emerging Global Diseases (CTEGD). “In many parts of the world, relapses account for the majority of total P. vivax cases.”
The announcement comes on the heels of reports of the first locally acquired cases of malaria in the United States in 20 years. In the summer of 2023, seven cases of locally acquired P. vivax malaria were reported in Sarasota, Fla., and one in Cameron County, Texas. These are in addition to a case of P. falciparum diagnosed in a Maryland resident living in the National Capital Region.
Most malaria cases diagnosed in the United States occur in people who have traveled to countries in South America, Africa, and southeast Asia where malaria is endemic. While locally acquired mosquito-transmitted malaria cases can occur, as Anopheles mosquito vectors exist throughout the United States, they are rare. The last reported outbreak was in 2003 when eight cases of locally acquired P. vivax malaria were identified in Palm Beach County, Fla.
The GHIT award will allow Maher and Chet Joyner to develop a compound series drug-screening program. Joyner is an assistant professor in the College of Veterinary Medicine’s Department of Infectious Diseases and Center for Vaccines and Immunology and jointly appointed to CTEGD.
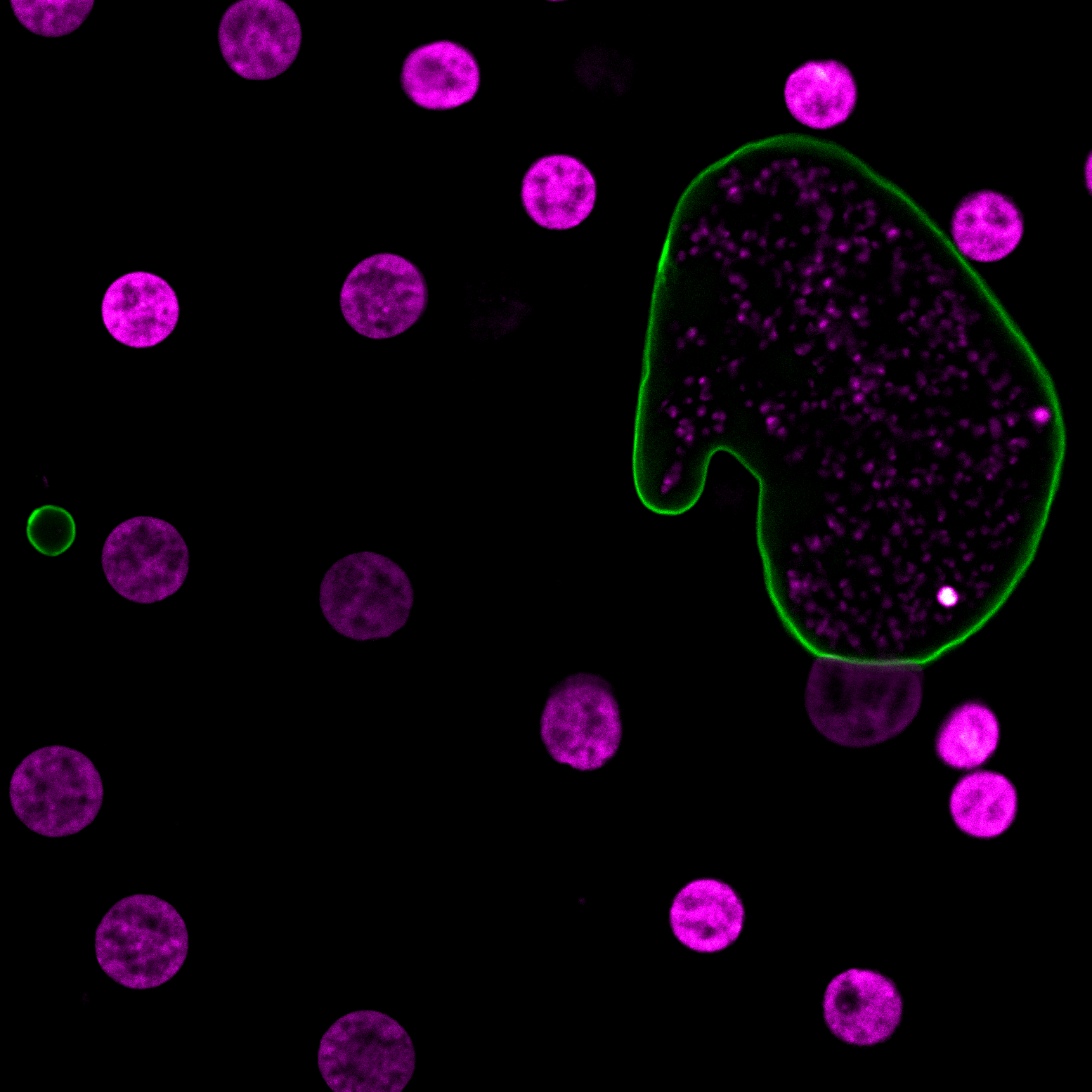
The compound series identified by Maher, the result of testing more than 100,000 samples using infected liver cells, is the first new chemical class discovered in more than 70 years with efficacy against the persisting liver stage. Over the next two years, Maher and Joyner will be collaborating with Medicine for Malaria Venture and Mitsubishi Tanabe Pharma Corporation to alter the chemistry of the compound to improve drug-like properties, including half-life and potency, necessary to achieve single dose criteria.
“Discovering a drug to kill dormant, non-proliferating cells is extremely difficult, yet with the novel assay the team developed we now have the first new target and drug class with potential to accelerate global malaria elimination efforts,” said Dennis Kyle, director of the CTEGD.
The current drug class used to treat P. vivax malaria, 8-aminoquinolines, often results in serious side effects and cannot be administered to pregnant women, who are one of the patient groups most in need of treatment.
“We have the first validated compound that kills vivax while it lies dormant in the liver,” Joyner said. “We hope in the next two years to help advance the new compounds to clinical testing.”
Lisa K. Nolan, dean of the College of Veterinary Medicine, said the work Maher and Joyner are doing could deliver a better quality of life to millions of people around the world.
“This great research is a shining example of our commitment to translational research, which will take this drug from the lab to preclinical testing to the patient rapidly,” Nolan said.