Oxidative Stress Contributes to Poor Pregnancy Outcomes During Placental Malaria
Julie Moore and her colleagues at the University of Georgia have determined that oxidative stress plays a role in the poor pregnancy outcomes that occur during placental malaria. Furthermore, they have identified the antioxidant tempol (TPL) as a potential therapeutic treatment.
What is placental malaria?
Placental Malaria is characterized by sequestration of Plasmodium falciparum, the parasite that causes malaria, in the maternal placental blood space. The ensuing damage to the placenta results in complications such as dangerously low birth weight, spontaneous abortion, and stillbirth. About 200,000 infants die annually as a result of placental malaria.
Pregnancy causes women to be more susceptible to malarial infection; however, it is an area of malarial research that has been long neglected. Julie Moore and her research team have studied the effects of malaria on pregnancy for more than 20 years.
Oxidative Stress is a contributor to poor pregnancy outcomes
Oxidative stress is an imbalance between the production of free radicals and the ability of the body to counteract or detoxify their harmful effects through the neutralization by antioxidants.
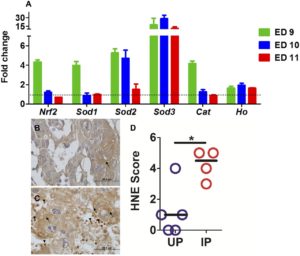
Evidence of oxidative stress in human placental malaria was observed through immunohistochemical detection of lipid peroxidation, which occurs in the presence of oxygen radicals. This observation motivated mechanistic studies of malaria-induced oxidative stress in a mouse model. Other researchers have determined that oxidative stress contributes to pathogenesis for another Plasmodium parasite. Based on those findings, Moore hypothesized that oxidative stress participates in the pathogenesis of infection with Plasmodium chabaudi AS, a murine infective parasite, during early pregnancy.
Oxidative stress in mice was measured by quantitative PCR of markers of an antioxidant response and by immunochemistry of lipid peroxidation. Because oxidative stress and elevated free radicals are also associated with inflammation in both humans and mice, cytokine levels in antioxidant-treated and untreated animals were measured.
Their findings support their hypothesis that oxidative stress contributes to poor pregnancy outcomes during placental malaria. Furthermore, their findings corroborate studies by other research groups that malaria impacts the antioxidant response in the placenta and promotes oxidative stress.
Antioxidant tempol (TPL) is a potential therapeutic to treat oxidative stress in placental malaria
In their study, the Moore group looked at two possible antioxidants to combat the effects of oxidative stress on placental malaria pregnancy in mice.
First, they looked at N-acetylcysteine (NAC). The results indicate that, at the dose used in this study, NAC failed to improve pregnancy outcome in P. chabaudi AS-infected mice. In fact, 50% of NAC-treated infected mice aborted earlier than the control group of infected mice. Due to the possible toxicity of NAC, they did not further explore the use of NAC.
Next, they turned to TPL, a superoxide dismutase mimetic, that has been studied extensively in animal models of increased free radicals and oxidative stress.
While the intraperitoneally delivered TPL did not impact the outcome of infections in terms of weight change, anemia, or course of parasitemia, infected TPL-treated mice did show a significantly higher proportion of viable embryos compared to the untreated infected mice.
Following this promising outcome, the group next tested if constant administration via drinking water could improve the outcome. They found the proportion of viable embryos in infected TPL-treated mice was significantly higher than in the untreated infected mice. TPL efficacy was variable at the placental level; embryo viability was highest in those animals with TPL-mediated reduction in placental lipid peroxidation.
Their results indicate that TPL delivered orally ad libitum has a limited but significant positive effect on embryo survival in infected mice.
They tentatively conclude, in the absence of confirmation of TPL in plasma or urine, bioavailability for TPL in the placenta is poor, but in cases in which it can reach effective local concentrations, oral TPL can suppress placental lipid peroxidation and positively impact embryo outcome.
Areas for more research
The findings of this study have opened new avenues of research that need to be explored.
- In placental malaria, where sequestered parasites and responding immune cells are likely to represent a potent source of free radicals, extracellular superoxide dismutase may be critical to counterbalance high levels of oxidative stress. Further studies will be required to determine the extent to which this particular enzyme may be a critical player in the response to placental malaria, as well as cellular sources.
- In their analysis of oxidative damage and TPL efficacy, they also detected lipid peroxidation. Only lipid peroxidation generated in situ by endogenous free radicals would be targeted by TPL, but peroxidized lipids can be contributed exogenously, such as by components of the malaria parasite. It is therefore plausible that parasite-derived pro-oxidant effects may limit NAC and TPL efficacy. Future studies in their model and others will be necessary to confirm the sources and contribution of lipid peroxidation to malaria pathogenesis.
- Because inflammatory responses are critical in placental malaria pathogenesis, the extent to which these responses are altered was a logical consideration in TPL-treated mice. Just as TPL efficacy in reducing placental lipid peroxidation was variable in malaria-infected pregnant mice, so was its ability to alter systemic inflammatory responses. An indicator of TPL efficacy might be its ability to suppress inflammatory cytokine and chemokine production. Future studies of antioxidant therapy in infected mice will need to resolve the relative contributions of TPL-mediated antioxidant and anti-inflammatory effects.
The full article is available online: Demba Sarr, Caitlin A. Cooper, Tara C. Bracken, Omar Martinez-Uribe, Tamas Nagy and Julie M. Moore. Oxidative Stress: A Potential Therapeutic Target in Placental Malaria. ImmunoHorizons June 1, 2017, 1 (4) 29-41; DOI: https://doi.org/10.4049/immunohorizons.1700002
