Massive invasion of organellar DNA drives nuclear genome evolution in Toxoplasma
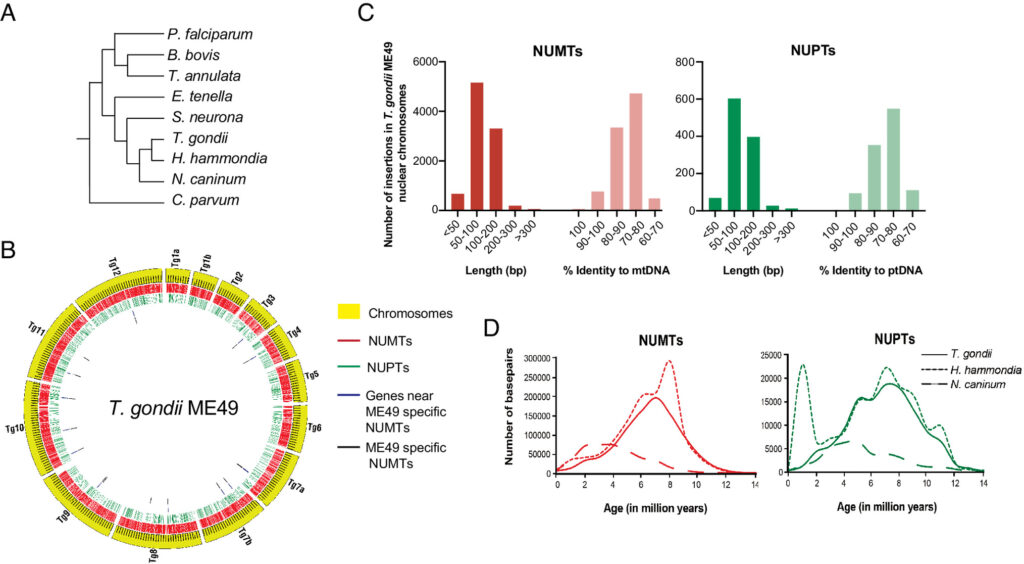
Toxoplasma gondii is a zoonotic protist pathogen that infects up to one third of the human population. This apicomplexan parasite contains three genome sequences: nuclear (65 Mb); plastid organellar, ptDNA (35 kb); and mitochondrial organellar, mtDNA (5.9 kb of non-repetitive sequence). We find that the nuclear genome contains a significant amount of NUMTs (nuclear integrants of mitochondrial DNA) and NUPTs (nuclear integrants of plastid DNA) that are continuously acquired and represent a significant source of intraspecific genetic variation. NUOT (nuclear DNA of organellar origin) accretion has generated 1.6% of the extant T. gondii ME49 nuclear genome-the highest fraction ever reported in any organism. NUOTs are primarily found in organisms that retain the non-homologous end-joining repair pathway. Significant movement of organellar DNA was experimentally captured via amplicon sequencing of a CRISPR-induced double-strand break in non-homologous end-joining repair competent, but not ku80 mutant, Toxoplasma parasites. Comparisons with Neospora caninum, a species that diverged from Toxoplasma ~28 mya, revealed that the movement and fixation of five NUMTs predates the split of the two genera. This unexpected level of NUMT conservation suggests evolutionary constraint for cellular function. Most NUMT insertions reside within (60%) or nearby genes (23% within 1.5 kb), and reporter assays indicate that some NUMTs have the ability to function as cis-regulatory elements modulating gene expression. Together, these findings portray a role for organellar sequence insertion in dynamically shaping the genomic architecture and likely contributing to adaptation and phenotypic changes in this important human pathogen.
Sivaranjani Namasivayam, Cheng Sun, Assiatu B Bah, Jenna Oberstaller, Edwin Pierre-Louis, Ronald Drew Etheridge, Cedric Feschotte, Ellen J Pritham, Jessica C Kissinger. Proc Natl Acad Sci U S A. 2023 Nov 7;120(45):e2308569120. doi: 10.1073/pnas.2308569120.


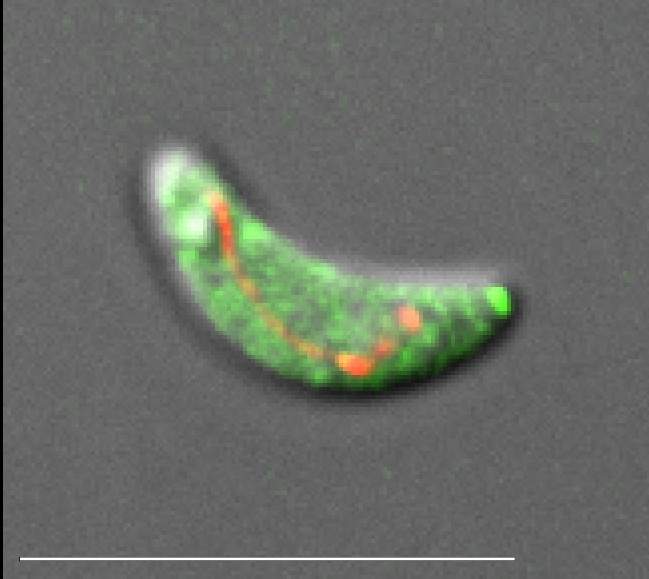
 Vacuolar-proton ATPases (V-ATPases) are conserved complexes that couple the hydrolysis of ATP to the pumping of protons across membranes. V-ATPases are known to play diverse roles in cellular physiology. We studied the Toxoplasma gondiiV-ATPase complex and discovered a dual role of the pump in protecting parasites against ionic stress and in the maturation of secretory proteins in endosomal-like compartments. Toxoplasma V-ATPase subunits localize to the plasma membrane and to acidic vesicles, and characterization of conditional mutants of the a1 subunit highlighted the functionality of the complex at both locations. Microneme and rhoptry proteins are required for invasion and modulation of host cells, and they traffic via endosome-like compartments in which proteolytic maturation occurs. We show that the V-ATPase supports the maturation of rhoptry and microneme proteins, and their maturases, during their traffic to their corresponding organelles. This work underscores a role for V-ATPases in regulating virulence pathways.
Vacuolar-proton ATPases (V-ATPases) are conserved complexes that couple the hydrolysis of ATP to the pumping of protons across membranes. V-ATPases are known to play diverse roles in cellular physiology. We studied the Toxoplasma gondiiV-ATPase complex and discovered a dual role of the pump in protecting parasites against ionic stress and in the maturation of secretory proteins in endosomal-like compartments. Toxoplasma V-ATPase subunits localize to the plasma membrane and to acidic vesicles, and characterization of conditional mutants of the a1 subunit highlighted the functionality of the complex at both locations. Microneme and rhoptry proteins are required for invasion and modulation of host cells, and they traffic via endosome-like compartments in which proteolytic maturation occurs. We show that the V-ATPase supports the maturation of rhoptry and microneme proteins, and their maturases, during their traffic to their corresponding organelles. This work underscores a role for V-ATPases in regulating virulence pathways.