Strain-specific genome evolution in Trypanosoma cruzi, the agent of Chagas disease
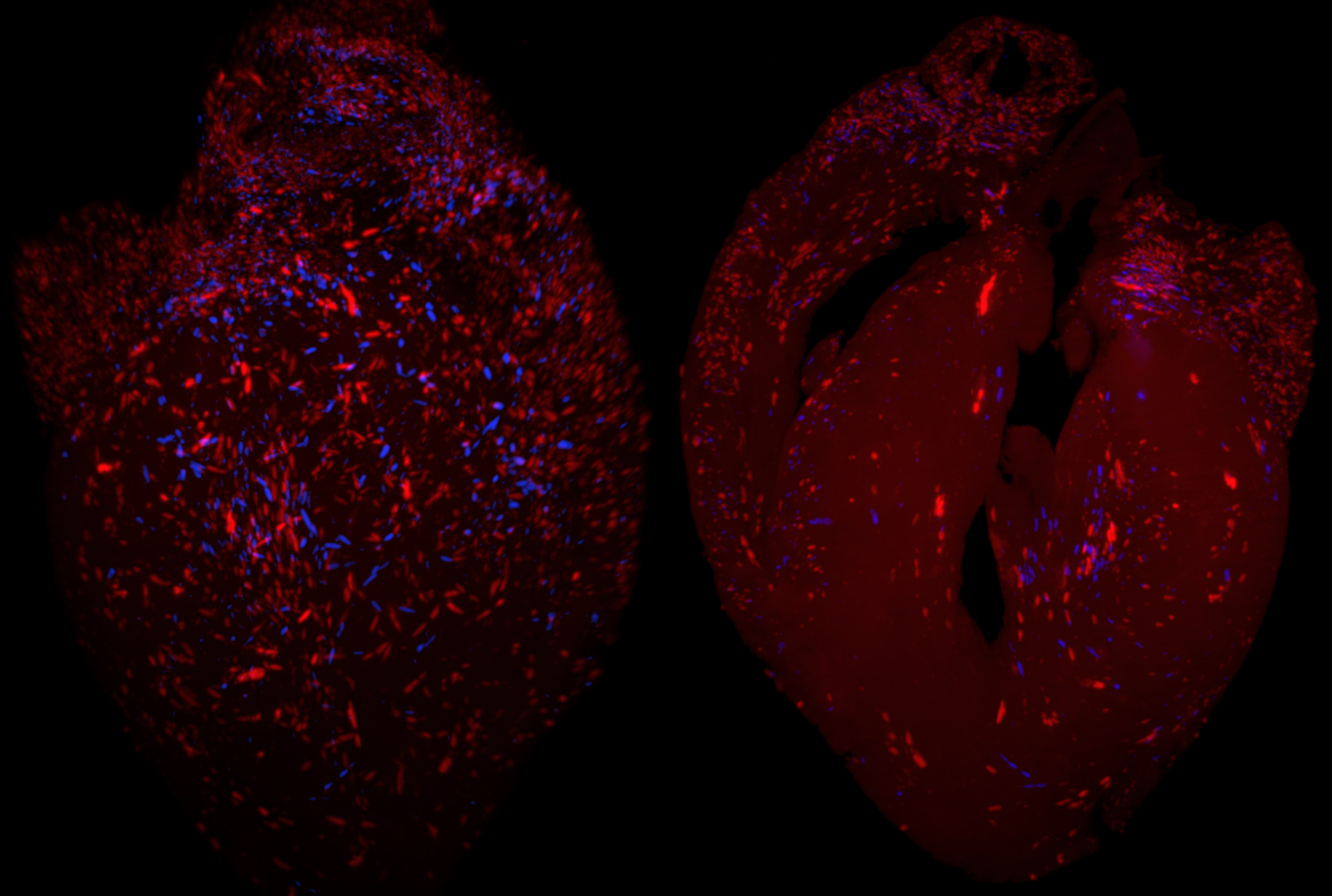
The protozoan Trypanosoma cruzi almost invariably establishes life-long infections in humans and other mammals, despite the development of potent host immune responses that constrain parasite numbers. The consistent, decades-long persistence of T. cruzi in human hosts arises at least in part from the remarkable level of genetic diversity in multiple families of genes encoding the primary target antigens of anti-parasite immune responses. However, the highly repetitive nature of the genome-largely a result of these same extensive families of genes-have prevented a full understanding of the extent of gene diversity and its maintenance in T. cruzi. In this study, we have combined long-read sequencing and proximity ligation mapping to generate very high-quality assemblies of two T. cruzi strains representing the apparent ancestral lineages of the species. These assemblies reveal not only the full repertoire of the members of large gene families in the two strains, demonstrating extreme diversity within and between isolates, but also provide evidence of the processes that generate and maintain that diversity, including extensive gene amplification, dispersion of copies throughout the genome and diversification via recombination and in situ mutations. Gene amplification events also yield significant copy number variations in a substantial number of genes presumably not required for or involved in immune evasion, thus forming a second level of strain-dependent variation in this species. The extreme genome flexibility evident in T. cruzi also appears to create unique challenges with respect to preserving core genome functions and gene expression that sets this species apart from related kinetoplastids.
Wang W, Peng D, Baptista RP, Li Y, Kissinger JC, Tarleton RL (2021) Strain-specific genome evolution in Trypanosoma cruzi, the agent of Chagas disease. PLoS Pathog 17(1): e1009254. https://doi.org/10.1371/journal.ppat.1009254







 Dr. Fernando Sanchez-Valdéz, from Salta, Argentina, completed a Ph.D. in Molecular Biology at the Faculty of Pharmacy and Biochemistry at the University of Buenos Aires, Argentina in 2014. After his Ph.D., he completed a postdoctoral fellowship in
Dr. Fernando Sanchez-Valdéz, from Salta, Argentina, completed a Ph.D. in Molecular Biology at the Faculty of Pharmacy and Biochemistry at the University of Buenos Aires, Argentina in 2014. After his Ph.D., he completed a postdoctoral fellowship in