Reciprocal interactions between neuropeptide F and RYamide regulate host attraction in the mosquito Aedes aegypti
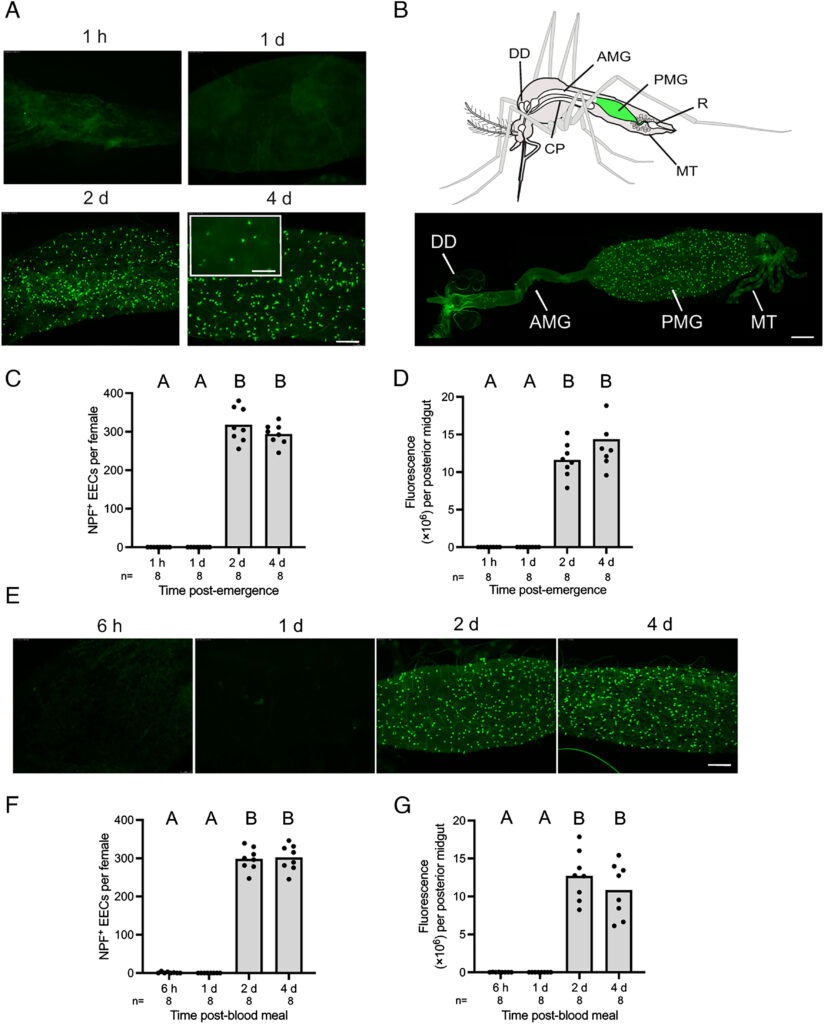
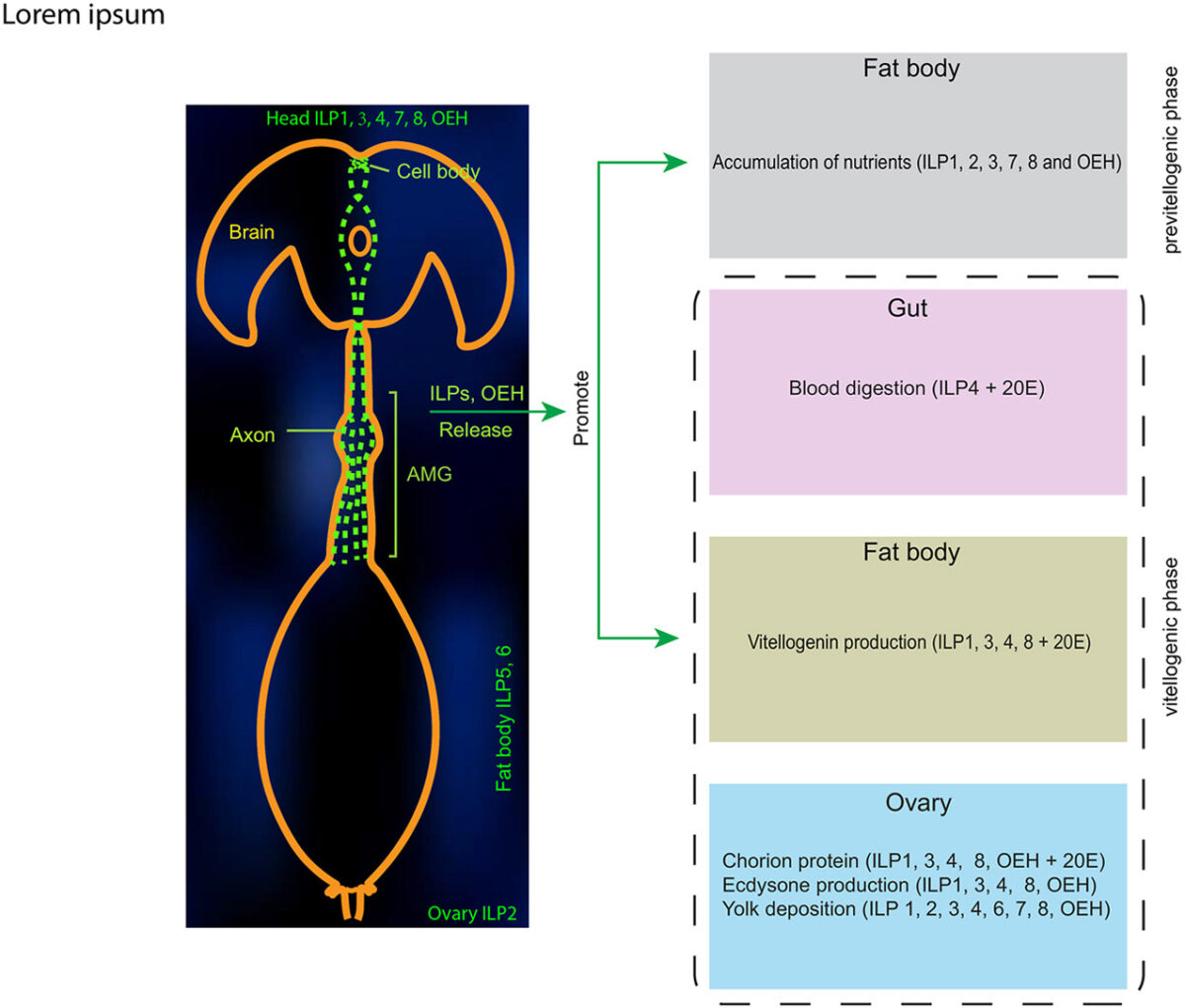
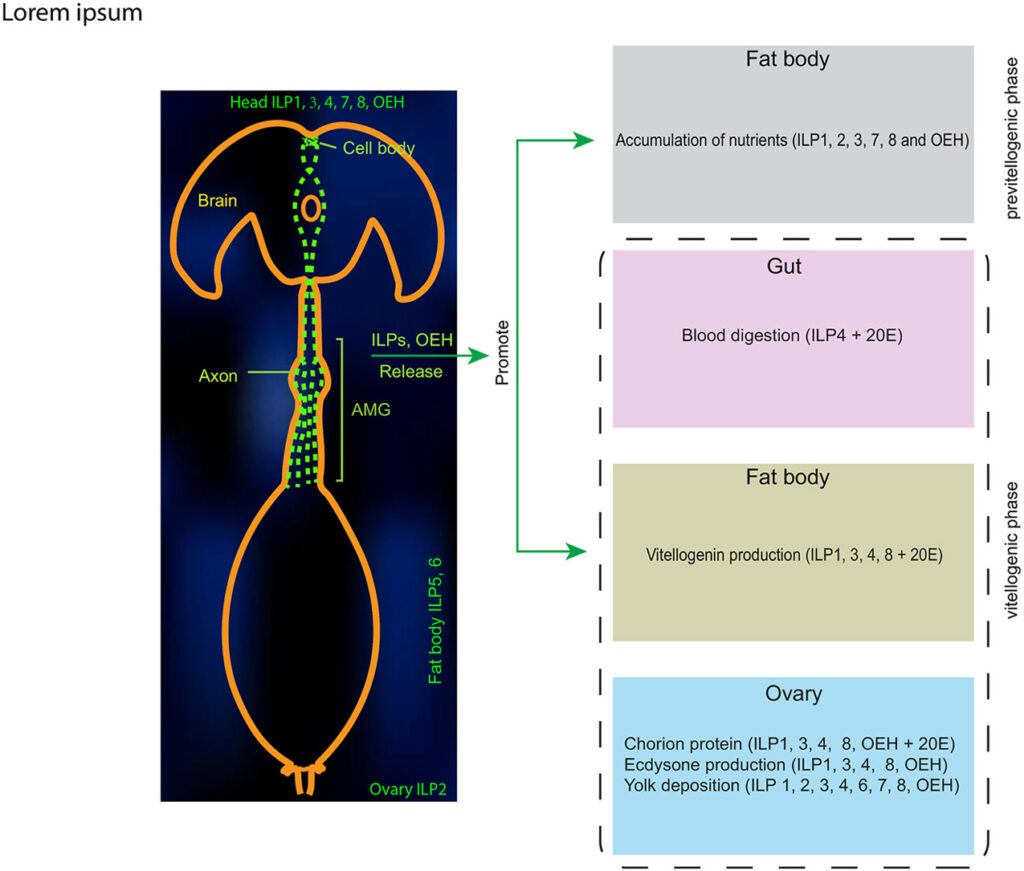
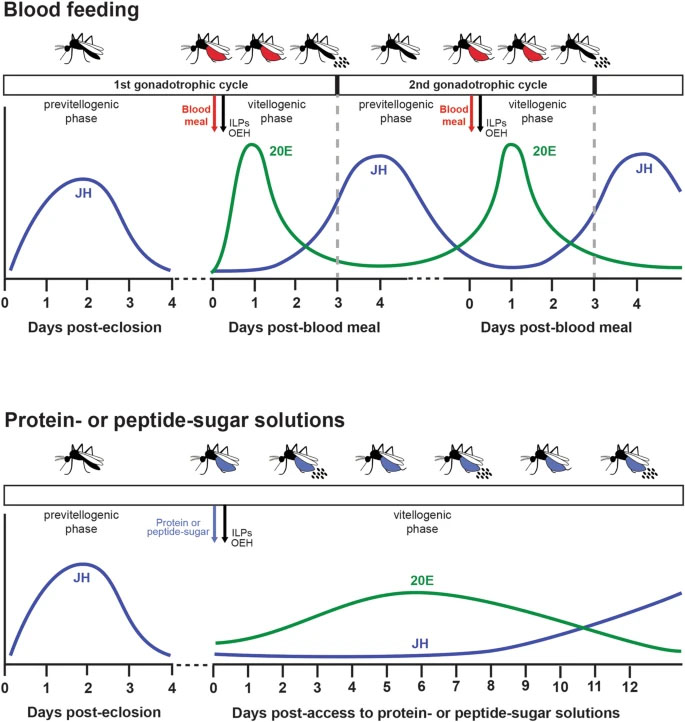
Female mosquitoes produce eggs in gonadotrophic cycles that are divided between a previtellogenic and vitellogenic phase. Previtellogenic females consume water and sugar sources like nectar while also being attracted to hosts for blood feeding. Consumption of a blood meal activates the vitellogenic phase, which produces mature eggs and suppresses host attraction. In this study, we tested the hypothesis that neuropeptide Y-like hormones differentially modulate host attraction behavior in the mosquito Aedes aegypti. A series of experiments collectively indicated that enteroendocrine cells (EECs) in the posterior midgut produce and release neuropeptide F (NPF) into the hemolymph during the previtellogenic phase which stimulates attraction to humans and biting behavior. Consumption of a blood meal, which primarily consists of protein by dry weight, down-regulated NPF in EECs until mature eggs developed, which was associated with a decline in hemolymph titer. NPF depletion depended on protein digestion but was not associated with EEC loss. Other experiments showed that neurons in the terminal ganglion extend axons to the posterior midgut and produce RYamide, which showed evidence of increased secretion into circulation after a blood meal. Injection of RYamide-1 and -2 into previtellogenic females suppressed host attraction, while coinjection of RYamides with or without short NPF-2 also inhibited the host attraction activity of NPF. Overall, our results identify NPF and RYamide as gut-associated hormones in A. aegypti that link host attraction behavior to shifts in diet during sequential gonadotrophic cycles.
Xiaoyi Dou, Kangkang Chen, Mark R Brown, Michael R Strand. Proc Natl Acad Sci U S A. 2024 Jul 9;121(28):e2408072121. doi: 10.1073/pnas.2408072121.