Synthesis and biological evaluation of 1-alkylaminomethyl-1,1-bisphosphonic acids against Trypanosoma cruzi and Toxoplasma gondii
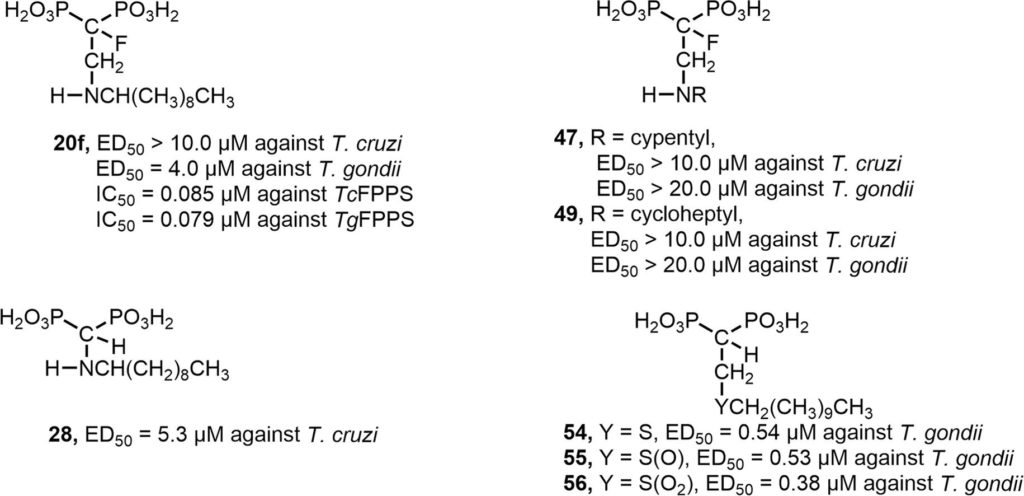
As an extension of our project aimed at the search for new chemotherapeutic agents against Chagas disease and toxoplasmosis, several 1,1-bisphosphonates were designed, synthesized and biologically evaluated against Trypanosoma cruzi and Toxoplasma gondii, the etiologic agents of these diseases, respectively. In particular, and based on the antiparasitic activity exhibited by 2-alkylaminoethyl-1,1-bisphosphonates targeting farnesyl diphosphate synthase, a series of linear 2-alkylaminomethyl-1,1-bisphosphonic acids (compounds 21–33), that is, the position of the amino group was one carbon closer to the gem-phosphonate moiety, were evaluated as growth inhibitors against the clinically more relevant dividing form (amastigotes) of T. cruzi. Although all of these compounds resulted to be devoid of antiparasitic activity, these results were valuable for a rigorous SAR study. In addition, unexpectedly, the synthetic designed 2-cycloalkylaminoethyl-1,1-bisphosphonic acids 47–49 were free of antiparasitic activity. Moreover, long chain sulfur-containing 1,1-bisphosphonic acids, such as compounds 54–56, 59, turned out to be nanomolar growth inhibitors of tachyzoites of T. gondii. As many bisphosphonate-containing molecules are FDA-approved drugs for the treatment of bone resorption disorders, their potential nontoxicity makes them good candidates to control American trypanosomiasis and toxoplasmosis.
Tamila Galaka, Bruno N. Falcone, Catherine Li, Sergio H. Szajnman, Silvia N.J. Moreno, Roberto Docampo, Juan B.Rodriguez. Bioorg Med Chem. 2019 Jul 4. pii: S0968-0896(19)30740-0. doi: 10.1016/j.bmc.2019.07.004.

 Cryptosporidium is a leading cause of diarrheal disease and an important contributor to early childhood mortality, malnutrition, and growth faltering. Older children in high endemicity regions appear resistant to infection, while previously unexposed adults remain susceptible. Experimental studies in humans and animals support the development of disease resistance, but we do not understand the mechanisms that underlie protective immunity to Cryptosporidium. Here, we derive an in vivo model of Cryptosporidium infection in immunocompetent C57BL/6 mice by isolating parasites from naturally infected wild mice. Similar to human cryptosporidiosis, this infection causes intestinal pathology, and interferon-γ controls early infection while T cells are critical for clearance. Importantly, mice that controlled a live infection were resistant to secondary challenge and vaccination with attenuated parasites provided protection equal to live infection. Both parasite and host are genetically tractable and this in vivo model will facilitate mechanistic investigation and rational vaccine design.
Cryptosporidium is a leading cause of diarrheal disease and an important contributor to early childhood mortality, malnutrition, and growth faltering. Older children in high endemicity regions appear resistant to infection, while previously unexposed adults remain susceptible. Experimental studies in humans and animals support the development of disease resistance, but we do not understand the mechanisms that underlie protective immunity to Cryptosporidium. Here, we derive an in vivo model of Cryptosporidium infection in immunocompetent C57BL/6 mice by isolating parasites from naturally infected wild mice. Similar to human cryptosporidiosis, this infection causes intestinal pathology, and interferon-γ controls early infection while T cells are critical for clearance. Importantly, mice that controlled a live infection were resistant to secondary challenge and vaccination with attenuated parasites provided protection equal to live infection. Both parasite and host are genetically tractable and this in vivo model will facilitate mechanistic investigation and rational vaccine design.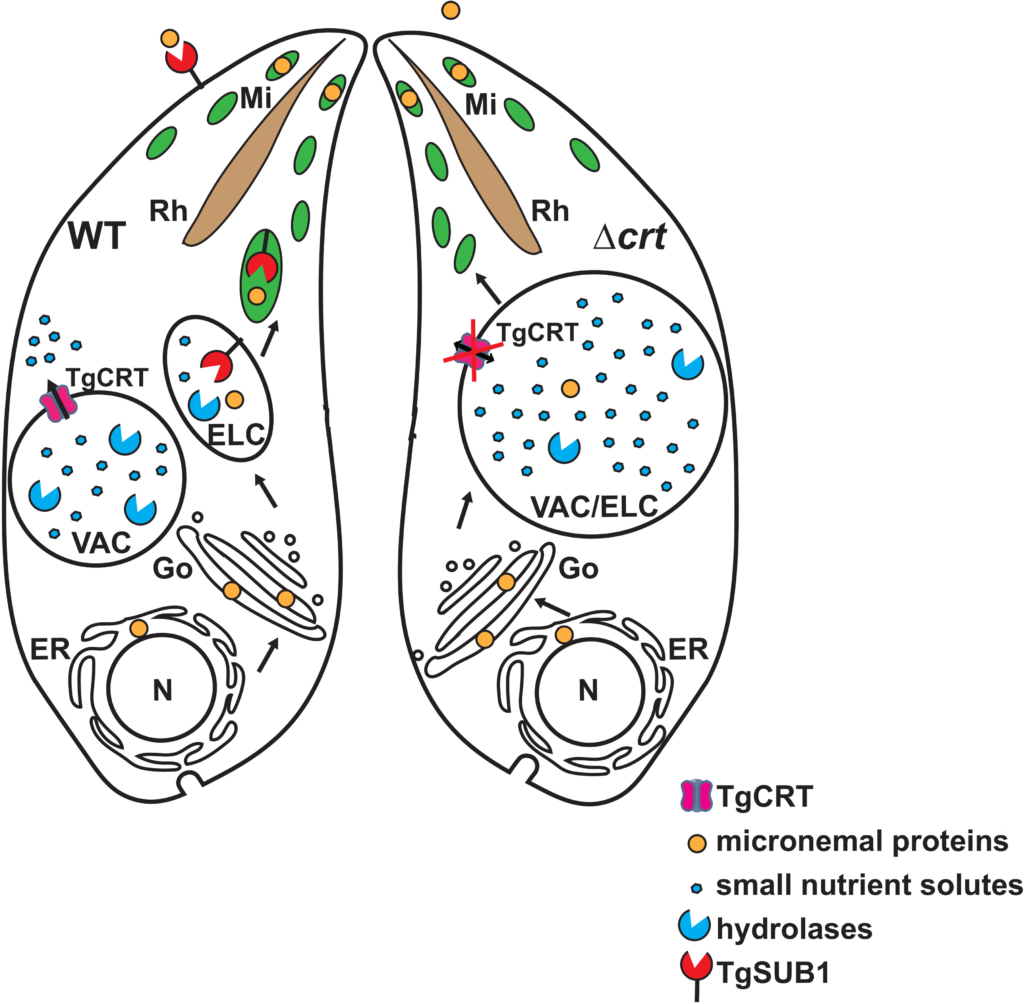 Toxoplasma gondii is an apicomplexan parasite with the ability to use foodborne, zoonotic, and congenital routes of transmission that causes severe disease in immunocompromised patients. The parasites harbor a lysosome-like organelle, termed the “Vacuolar Compartment/Plant-Like Vacuole” (VAC/PLV), which plays an important role in maintaining the lytic cycle and virulence of T. gondii. The VAC supplies proteolytic enzymes that contribute to the maturation of invasion effectors and that digest autophagosomes and endocytosed host proteins. Previous work identified a T. gondii ortholog of the Plasmodium falciparum chloroquine resistance transporter (PfCRT) that localized to the VAC. Here, we show that TgCRT is a membrane transporter that is functionally similar to PfCRT. We also genetically ablate TgCRT and reveal that the TgCRT protein plays a key role in maintaining the integrity of the parasite’s endolysosomal system by controlling morphology of the VAC. When TgCRT is absent, the VAC dramatically increases in volume by ~15-fold and overlaps with adjacent endosome-like compartments. Presumably to reduce aberrant swelling, transcription and translation of endolysosomal proteases are decreased in ΔTgCRT parasites. Expression of subtilisin protease 1 is significantly reduced, which impedes trimming of microneme proteins, and significantly decreases parasite invasion. Chemical or genetic inhibition of proteolysis within the VAC reverses these effects, reducing VAC size and partially restoring integrity of the endolysosomal system, microneme protein trimming, and invasion. Taken together, these findings reveal for the first time a physiological role of TgCRT in substrate transport that impacts VAC volume and the integrity of the endolysosomal system in T. gondii.
Toxoplasma gondii is an apicomplexan parasite with the ability to use foodborne, zoonotic, and congenital routes of transmission that causes severe disease in immunocompromised patients. The parasites harbor a lysosome-like organelle, termed the “Vacuolar Compartment/Plant-Like Vacuole” (VAC/PLV), which plays an important role in maintaining the lytic cycle and virulence of T. gondii. The VAC supplies proteolytic enzymes that contribute to the maturation of invasion effectors and that digest autophagosomes and endocytosed host proteins. Previous work identified a T. gondii ortholog of the Plasmodium falciparum chloroquine resistance transporter (PfCRT) that localized to the VAC. Here, we show that TgCRT is a membrane transporter that is functionally similar to PfCRT. We also genetically ablate TgCRT and reveal that the TgCRT protein plays a key role in maintaining the integrity of the parasite’s endolysosomal system by controlling morphology of the VAC. When TgCRT is absent, the VAC dramatically increases in volume by ~15-fold and overlaps with adjacent endosome-like compartments. Presumably to reduce aberrant swelling, transcription and translation of endolysosomal proteases are decreased in ΔTgCRT parasites. Expression of subtilisin protease 1 is significantly reduced, which impedes trimming of microneme proteins, and significantly decreases parasite invasion. Chemical or genetic inhibition of proteolysis within the VAC reverses these effects, reducing VAC size and partially restoring integrity of the endolysosomal system, microneme protein trimming, and invasion. Taken together, these findings reveal for the first time a physiological role of TgCRT in substrate transport that impacts VAC volume and the integrity of the endolysosomal system in T. gondii.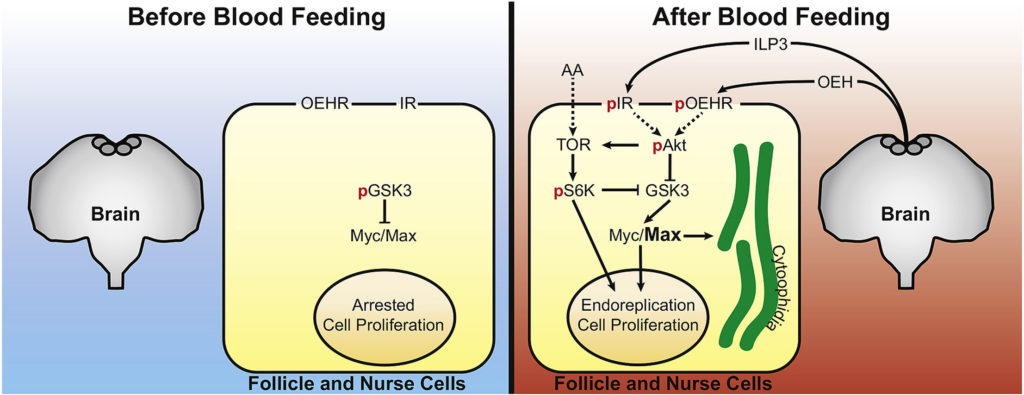 Most mosquitoes, including Aedes aegypti, only produce eggs after blood feeding on a vertebrate host. Oogenesis in A. aegypti consists of a pre-vitellogenic stage before blood feeding and a vitellogenic stage after blood feeding. Primary egg chambers remain developmentally arrested during the pre-vitellogenic stage but complete oogenesis to form mature eggs during the vitellogenic stage. In contrast, the signaling factors that maintain primary egg chambers in pre-vitellogenic arrest or that activate vitellogenic growth are largely unclear. Prior studies showed that A. aegypti females release insulin-like peptide 3 (ILP3) and ovary ecdysteroidogenic hormone (OEH) from brain neurosecretory cells after blood feeding. Here, we report that primary egg chambers exit pre-vitellogenic arrest by 8 h post-blood meal as evidenced by proliferation of follicle cells, endoreplication of nurse cells, and formation of cytoophidia. Ex vivo assays showed that ILP3 and OEH stimulate primary egg chambers to exit pre-vitellogenic arrest in the presence of nutrients but not in their absence. Characterization of associated pathways indicated that activation of insulin/insulin growth factor signaling (IIS) by ILP3 or OEH inactivated glycogen synthase kinase 3 (GSK3) via phosphorylation by phosphorylated Akt. GSK3 inactivation correlated with accumulation of the basic helix-loop-helix transcription factor Max and primary egg chambers exiting pre-vitellogenic arrest. Direct inhibition of GSK3 by CHIR-99021 also stimulated Myc/Max accumulation and primary egg chambers exiting pre-vitellogenic arrest. Collectively, our results identify GSK3 as a key factor in regulating the pre- and vitellogenic stages of oogenesis in A. aegypti.
Most mosquitoes, including Aedes aegypti, only produce eggs after blood feeding on a vertebrate host. Oogenesis in A. aegypti consists of a pre-vitellogenic stage before blood feeding and a vitellogenic stage after blood feeding. Primary egg chambers remain developmentally arrested during the pre-vitellogenic stage but complete oogenesis to form mature eggs during the vitellogenic stage. In contrast, the signaling factors that maintain primary egg chambers in pre-vitellogenic arrest or that activate vitellogenic growth are largely unclear. Prior studies showed that A. aegypti females release insulin-like peptide 3 (ILP3) and ovary ecdysteroidogenic hormone (OEH) from brain neurosecretory cells after blood feeding. Here, we report that primary egg chambers exit pre-vitellogenic arrest by 8 h post-blood meal as evidenced by proliferation of follicle cells, endoreplication of nurse cells, and formation of cytoophidia. Ex vivo assays showed that ILP3 and OEH stimulate primary egg chambers to exit pre-vitellogenic arrest in the presence of nutrients but not in their absence. Characterization of associated pathways indicated that activation of insulin/insulin growth factor signaling (IIS) by ILP3 or OEH inactivated glycogen synthase kinase 3 (GSK3) via phosphorylation by phosphorylated Akt. GSK3 inactivation correlated with accumulation of the basic helix-loop-helix transcription factor Max and primary egg chambers exiting pre-vitellogenic arrest. Direct inhibition of GSK3 by CHIR-99021 also stimulated Myc/Max accumulation and primary egg chambers exiting pre-vitellogenic arrest. Collectively, our results identify GSK3 as a key factor in regulating the pre- and vitellogenic stages of oogenesis in A. aegypti.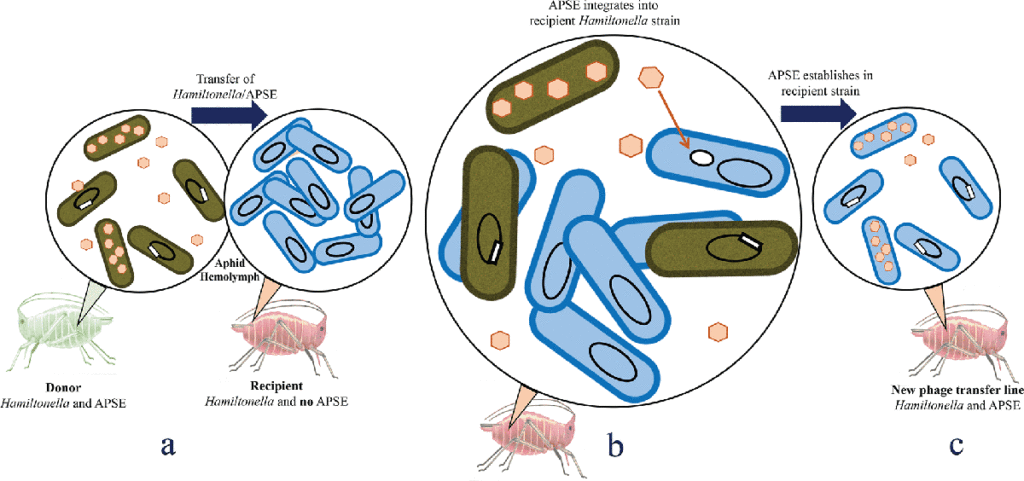 Insects are frequently infected with inherited facultative symbionts known to provide a range of conditionally beneficial services, including host protection. Pea aphids (Acyrthosiphon pisum) often harbour the bacterium
Insects are frequently infected with inherited facultative symbionts known to provide a range of conditionally beneficial services, including host protection. Pea aphids (Acyrthosiphon pisum) often harbour the bacterium