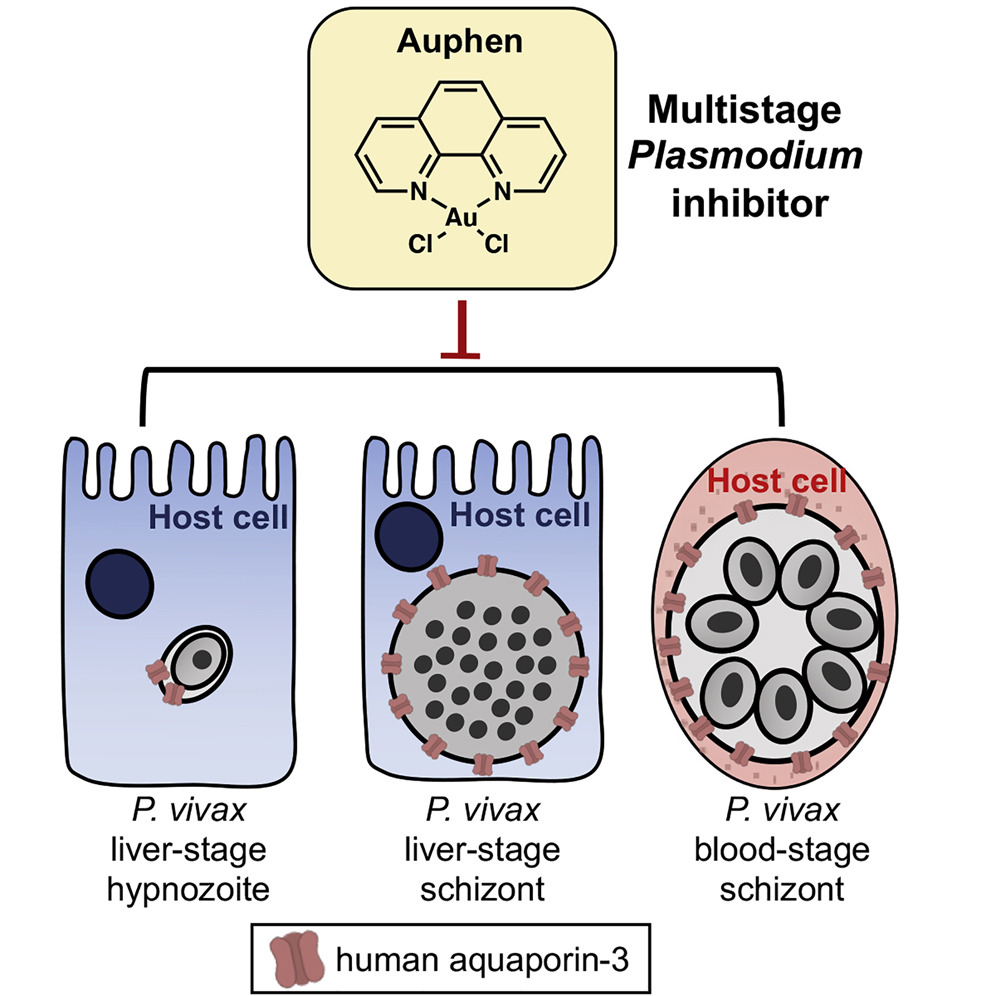
Plasmodium vivax Liver and Blood Stages Recruit the Druggable Host Membrane Channel Aquaporin-3
Plasmodium vivax infects hepatocytes to form schizonts that cause blood infection, or dormant hypnozoites that can persist for months in the liver before leading to relapsing blood infections. The molecular processes that drive P. vivax schizont and hypnozoite survival remain largely unknown, but they likely involve a rich network of host-pathogen interactions, including those occurring at the host-parasite interface, the parasitophorous vacuole membrane (PVM). Using a recently developed P. vivax liver-stage model system we demonstrate that host aquaporin-3 (AQP3) localizes to the PVM of schizonts and hypnozoites within 5 days after invasion. This recruitment is also observed in P. vivax-infected reticulocytes. Chemical treatment with the AQP3 inhibitor auphen reduces P. vivax liver hypnozoite and schizont burden, and inhibits P. vivax asexual blood-stage growth. These findings reveal a role for AQP3 in P. vivax liver and blood stages and suggest that the protein may be targeted for therapeutic treatment.
Dora Posfai, Steven P. Maher, Camille Roesch, Amélie Vantaux, Kayla Sylvester, Julie Péneau, Jean Popovici, Dennis E. Kyle, Benoît Witkowski, Emily R. Derbyshire. Cell Chem Biol. 2020 Mar 24. pii: S2451-9456(20)30083-0. doi: 10.1016/j.chembiol.2020.03.009.