Boris Striepen talks with NPR’s Joe Palca about cryptosporidium
Listen to the interview that aired on July 15 on All Things Considered.
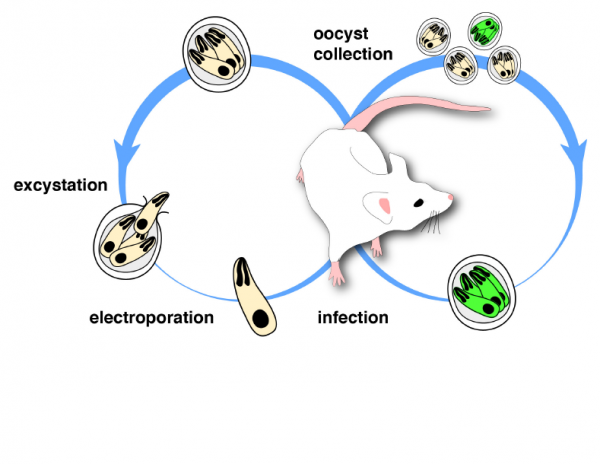
The diarrheal disease cyrptosporidiosis is the cause of 10.5% of child mortality globally. It is the second leading cause of diarrheal disease in sub-Sahara Africa and South Asia. It is a difficult parasite to study in the laboratory, which is why the recent technologies developed by the Striepen Lab are so important. These discoveries will impact the ability to develop new drug treatments and vaccines.
Learn more about the Striepen Lab.
